
Th c Hành Y H c Ch ng ự ọ ứ
C trong Y T Công C ng ứ ế ộ
Gs Ts Bs Lê Hoàng Ninh
Th c hành y t công ự ế
c ng d a trên ch ng cộ ự ứ ứ
Ki n th c/ ế ứ
nghiên c uứ
Th c tr ng b nh ự ạ ệ
nhân/ các tham
kh o ả
Kinh nghiêm
lâm sàng/ s ự
cân nh cắ

•“M t trong nh ng khám phá quan tr ng ộ ữ ọ
nh t, áng kính ng c nh t là bi t cái mà ấ đ ạ ấ ế
Ta có th làm và bi t s cái mà Ta không ể ế ợ
th làm.”ể
Henry Ford

M c tiêuụ
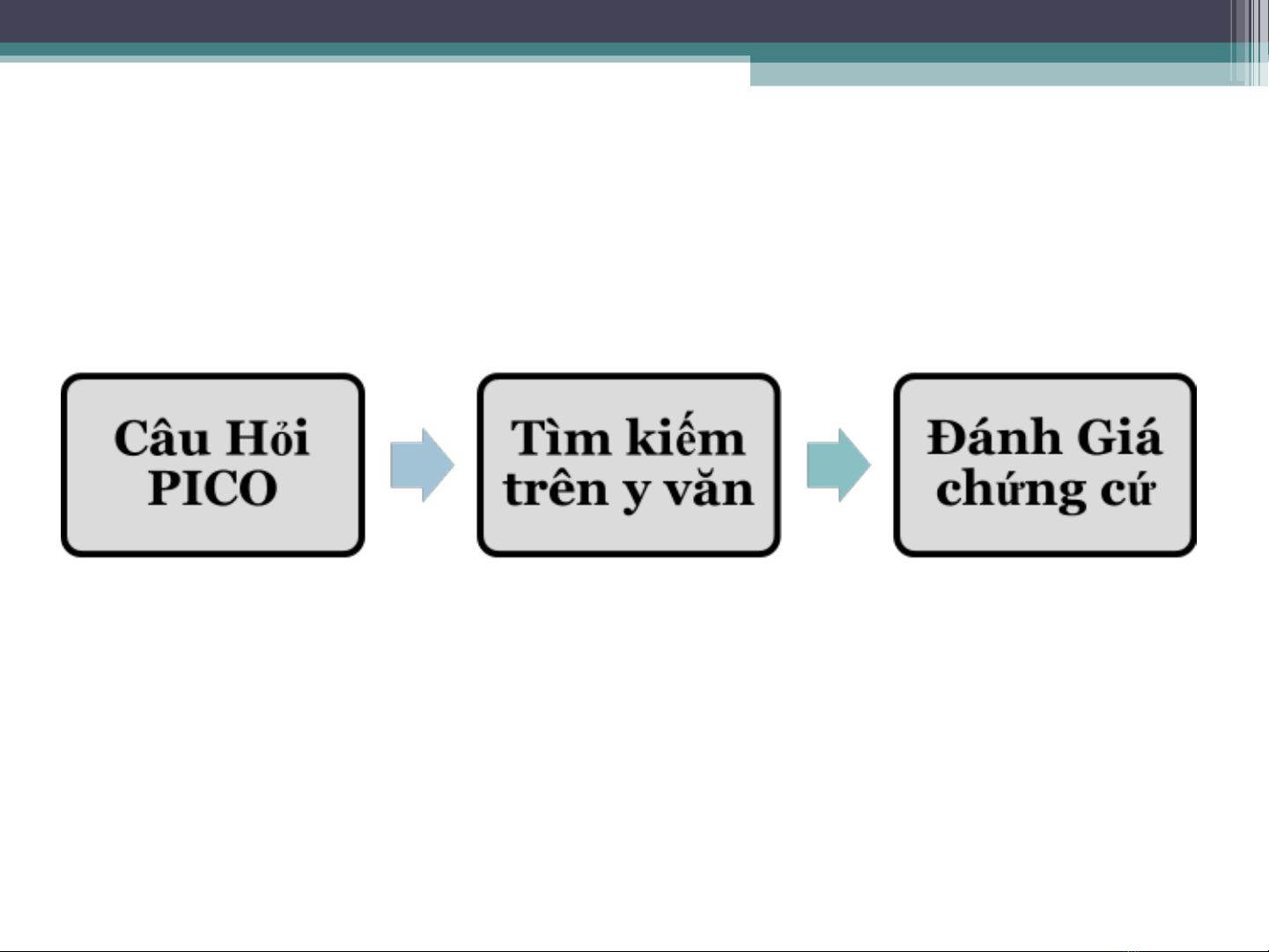
1)Hi u ý ngh a v ánh giá các ch ng c ể ĩ ề đ ứ ứ
2) Mô t m c ch ng c c dùng trong ả ứ độ ứ ứ đượ
ánh giá các ch ng cđ ứ ứ
3) Th m dò các ph ng pháp khác nh ă ươ ư
( th ng kê…) có th dùng trong ánh giá ố ể đ
ch ng c . ứ ứ
4) ng d ng qui trình n y trong y t công ứ ụ ầ ế
c ng.ộ


























