Can Tho Journal of Medicine and Pharmacy 9(6) (2023)
129
PHYTOCONSTITUENTS AND IN VITRO ANTI-UROLITHIATIC
ACTIVITY OF VARIOUS HYDROPHILIC EXTRACTS OF
TERMINALIA CATAPPA LEAVES ON CALCIUM OXALATE CRYSTAL
Vo Dang Thuan, Huynh Anh Duy*
College of Natural Sciences, Can Tho University
*Corresponding author: haduy@ctu.edu.vn
Received: 13/5/2023
Reviewed: 25/5/2023
Accepted: 19/09/2023
ABSTRACT
Background: Medicinal plants play an important role in the alternative or complement
therapy to manage of urinary stones at this time. Terminalia genus was proved anti-urolithiatic activity
via in vitro inhibition of calcium oxalate formation. Among the samples, Terminalia catappa showed
as a potential plants for this activity in India. Moreover, they were a common plant species in Vietnam
and there was no research on this topic in our country. Objectives: To evaluate in vitro anti-
urolithiatic activity of Terminalia catappa leaves in Vietnam, through inhibition of calcium oxalate
formation. In addition, moisture value, preliminary screening of the chemical composition and
determination of tanninoid content of aqueous extract also were conducted. Materials and methods:
Moisture content of herbs was conducted according to guidelines of 5th Vietnam pharmacopoeia,
appendix 9.6. Qualitation of the phytochemical constituents of aqueous extract with appropriate
reagents to confirm the presence of natural compounds via chemical reactions. Determination of
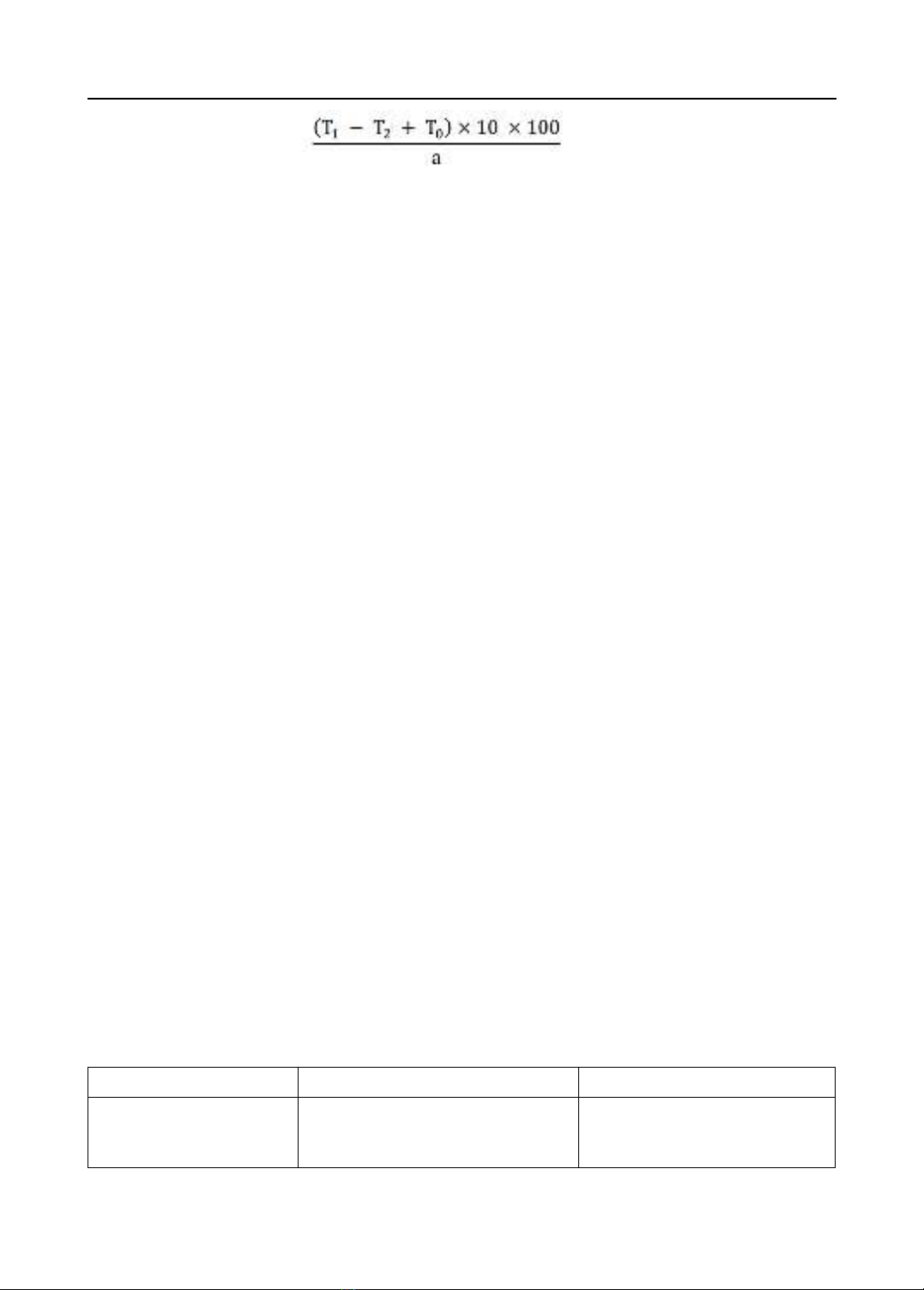
tanninoid content of aqueous extract was evaluated by using oxidation-reduction titration method
(Lowenthal assay) and skin powder method in instructing of appendix 12.6 from 5th Vietnam
pharmacopoeia. Inhibitory effect of calcium oxalate formation of three hydrophilic extracts (45%
ethanol, 96% ethanol and aqueous extracts) was confirmed by nucleation assay with cystone as a
positive control. Results: Moisture content of medicinal plant was 11.625%. Terminalia catappa
leaves contain the major phytochemical constituents such as flavonoids, tanninoids and saponins. The
tanninoid content according to Lowenthal method and skin powder method were 10.88% and 10.70%,
respectively. Therefore, the average tannin content was confirmed to be approximately 10.79%.
Among the investigated samples, aqueous extract showed the best inhibitory activity of calcium oxalate
crystal formation with an IC50 of 602.67 μg/mL when compared to cystone with IC50 423.05 μg/mL.
Conclusions: The aqueous extract from Terminalia catappa leaves has been shown to be a promising
source for anti-calcium oxalate crystals formation activity on experimental model. The anti-urolithic
potential of Terminalia catappa leaves may be related to its major phytoconstituents.
Keywords: Terminalia catappa, tanninoid, nucleation assay, calcium oxalate.
I. INTRODUCTION
Kidney stones (nephrolithiasis) were formed by the deposition of crystals in the
kidney. Urinary stone (urolithiasis) was a condition that occurs when these stones escaped
the kidney and moved into other part of the urinary system, including the ureters, bladder,
and urethra. These stones have been associated with urinary tract obstruction, kidney failure,
and urinary infections [1]. According to an epidemiological survey of urolithiasis in Asia,
the prevalence rate was 5-19.1% in West Asia, Southeast Asia, South Asia and some
developed countries (Korea and Japan). Meanwhile, this percentage was only 1-8% in most
of East Asia and North Asia. In addition, calcium oxalate was the most common kidney