Journal of Pharmaceutical Research and Drug Information 2025; 00(00); 000–000
_________________________________________
*Correspondence: vuongttt@hup.edu.vn; 1
http://doi.org/10.59882/1859-364X/264
Journal homepage: jprdi.vn/JP
Journal of Pharmaceutical Research and Drug Information
An official journal of Hanoi University of Pharmacy
Research article
Simultaneous determination of two UV filters
(octylmethoxycinnamate, octylsalicylate) in sunscreen creams
by high performance thin layer chromatography (HPTLC)
Minh Thuy Ngoa, Thi Bich Dao Danga, Dinh Chi Leb, Thi Thanh Vuong Tonga,*
a Department of Analytical Chemistry and Drug Quality Control, Hanoi University of
Pharmacy, 13-15 Le Thanh Tong Street, Hoan Kiem District, Hanoi, Vietnam
b National Institute of Pharmaceutical Technology, Hanoi University of Pharmacy, 13-15 Le
Thanh Tong Street, Hoan Kiem District, Hanoi, Vietnam.
*Corresponding author: Thi Thanh Vuong Tong - vuongttt@hup.edu.vn
Article history:
Received 05 December 2024
Resived 10 January 2025
Accepted 17 January 2025
ABSTRACT
Octylmethoxycinnamate (OMC), octylsalicylate (OS) are UV filters commonly used in
sunscreen creams for skin protection against UV radiations. In this study, an HPTLC
method was developed and validated for simultaneous determination of OMC and OS in
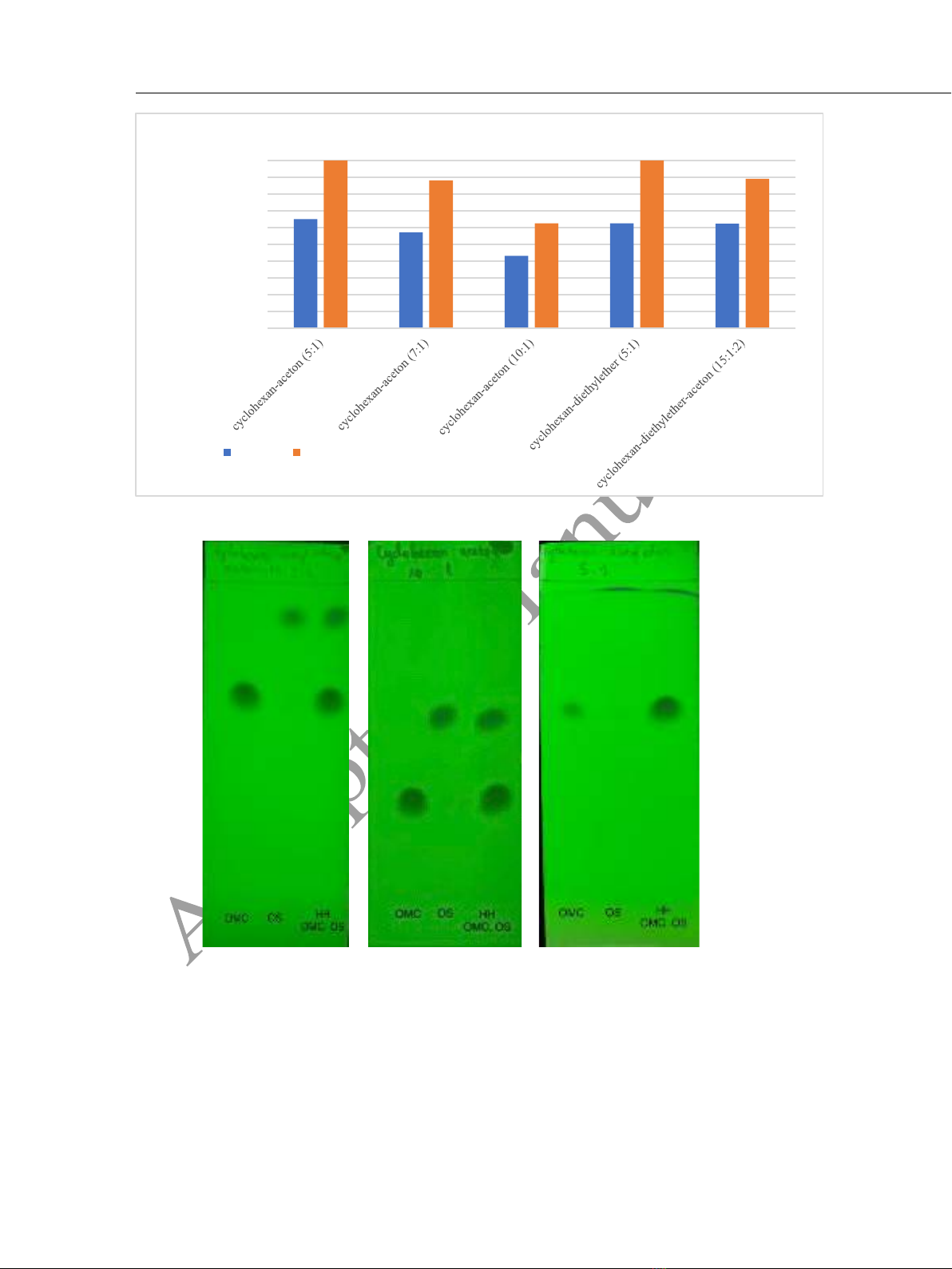
sunscreen creams. The method was carried out using an silica gel 60 F254 thin layer plate
(20 cm × 10 cm) with a mixture of cyclohexane - aceton (10:1, v/v) as mobile phase.
Samples were applied on thin layer plate as 4-mm bands with applied volume at 7.0 µL per
band and the distance between two consecutive bands was 9 mm. The chromatograms
were developed over a distance of 80mm, then scanned and recorded at 307 nm for peak
detection and calculation of peak area. The method was fully validated according to
requirements on analytical method performance of AOAC International. The linearity
ranges of the method were from 17.9 to 32.2 µg/mL for OMC and from 16.3 to 29.4
µg/mL for OS, respectively, and its accuracy (recovery rate from 97.4% to 102.2% for