
158 Monitoring in Rural Areas
this means that the system is not as fully automated as some might hope and that
regular visits to the stations by employees should be foreseen. Also, a too complex
‘black-box’ concept of the system leads to a significant loss of data. The processing
of the sensor signal to data should be transparent showing what can be done by using
PC-based modules for the control of the station. A web-based communication enables
remote control of the stations and the integration of the data into databases. This
concept also allows for a full remote control of the station by authorised persons and
a limited accessibility for data consultation by users through the web. A better spatial
representation can be obtained by embedding the monitoring and the modelling in a
GIS system (Vivoni and Richards, 2005).
2.3.4 CONCLUSIONS AND PERSPECTIVES
Monitoring in rural areas needs a different approach than in urban areas. The pollu-
tion in rural areas cannot be measured at certain points along the water body, but can
only be estimated by making evaluations of the water quality together with infor-
mation on what and how many polluting substances are applied in the area. Models,
describing all processes on those substances before entering the water, can provide
a means to evaluate the magnitude of pollution coming from diffuse pollution and
to evaluate scenarios for diffuse pollution reduction. Specific data are needed to
calibrate and build those models.
Therefore, the traditional cycle in water management should be inversed. Instead
of starting from the data set to select an appropriate tool and hence use this tool for
management, one should first define the problem, select a tool that can support this
problem and then design an appropriate monitoring program to feed the tool. In that
way, money is spent to generate primarily the information that is indeed needed. A
closer cooperation between monitoring and modelling efforts will make sure that
models for diffuse pollution can be used with sufficient reliability.
Automated monitoring can help to catch the high variability or short rain-driven
events. Such tools can only provide reliable data provided that the monitoring system
is transparent and follows quality control procedures with regard to maintenance and
calibration. While a high level of automation may support such procedures, it still
requires considerable manpower that should be foreseen in any monitoring budget.
REFERENCES
Arnold, J.G., Williams, J.R., Srinivasan, R. and King, K.W. (1996) SWAT Manual. USDA, Agri-
cultural Research Service and Blackland Research Center, Texas.
Barthelemy, P.A. and Vidal, C. (1999) A dynamic European agricultural and agri-foodstuff sec-
tor. In: Agriculture, Environment, Rural Development, Facts and Figures – A Challenge for
Agriculture. European Commission Report, Belgium.
Beck, M.B. (1987) Water Resour. Res.,23(8), 1393.

References 159
Bervoets, L., Schneiders, A. and Verheyen, R.F. (1989) Onderzoek naar de verspreiding en de
typologie van ecologisch waardevolle waterlopen in het Vlaams gewest. Deel 1 - Het Dender-
bekken, Universitaire Instelling Antwerpen. In Dutch.
Boschma, M., Joaris, A. and Vidal, C. (1999) Concentrations of livestock production. In: Agri-
culture, Environment, Rural Development, Facts and Figures – A Challenge for Agriculture.
European Commission Report, Belgium.
Brown, L.C. and Barnwell T.O. (1987) The Enhanced Stream Water Quality Models QUAL2E and
QUAL2E-UNCAS: Documentation and User Model. EPA/600/3-87/007, USA.
Janssen, P.H.M., Heuberger, P.S.C. and Sanders, S. (1992) Manual Uncsam 1.1, a Software Package
for Sensitivity and Uncertainty Analysis. Bilthoven, The Netherlands.
Krysanova, V. and Haberlandt, U. (2001) Ecol. Modelling,150, 255–275.
McKay, M.D. (1988) Sensitivity and uncertainty analysis using a statistical sample of input values.
In: Uncertainty Analysis, Y. Ronen, ed. CRC Press, Inc., Boca Raton, FL, pp. 145–186.
Montarella, L. (1999) Soil at the interface between agriculture and environment. In: Agriculture,
Environment, Rural Development, Facts and Figures – A Challenge for Agriculture. European
Commission Report, Belgium.
Pau Val, M. and Vidal, C. (1999) Nitrogen in agriculture. In: Agriculture, Environment, Rural
Development, Facts and Figures – A Challenge for Agriculture. European Commission Report,
Belgium.
Poirot, M. (1999) Crop trends and environmental impacts. In: Agriculture, Environment, Rural
Development, Facts and Figures – A Challenge for Agriculture. European Commission Report,
Belgium.
Sevruk, B. (1986) Proceedings of the ETH, IAHS International Workshop on the Correction of
Precipitation Measurements, 1–3 April 1985. ETH Z¨urich, Z¨uricher Geographische Schriften,
Z¨urich, p. 23.
Smets, S. (1999) Modelling of nutrient losses in the Dender catchment using SWAT. Masters
dissertation. Katholieke Universiteit Leuven –Vrije Universiteit, Brussels, Belgium.
Vandenberghe, V., van Griensven, A. and Bauwens, W. (2005) Water Sci.Technol., 51(3-4), 347–
354.
Vandenberghe, V., Goethals, P., van Griensven, A., Meirlaen, J., De Pauw, N., Vanrolleghem, P.A.
and Bauwens, W. (2004) Environ. Monitor Assess.,108, 85–98.
van Griensven, A. and Bauwens, W. (2001) Water Sci. Technol.,43(7), 321–328.
van Griensven, A. and Bauwens, W. (2003) Water Resour. Res.,39(10), 1348.
van Griensven, A., Vandenberghe, V. and Bauwens, W. (2002) Proceedings of the International
IWA Conference on Automation in Water Quality Monitoring, 21–22, May 2002. Vienna,
Austria.
Vivoni, E.R. and Richards, K.T. (2005) J. Hydroinform.,7(4), 235–250.

3.1
Elements of Modelling and
Control of Urban Wastewater
Treatment Systems
Olivier Potier and Marie-No¨elle Pons
3.1.1 Introduction
3.1.2 Short Description of the Biological Process by Activated Sludge
3.1.3 Process Parameters
3.1.3.1 Biokinetics
3.1.3.2 Oxygen Transfer
3.1.3.3 Hydrodynamics
3.1.3.4 Wastewater Variability
3.1.3.5 Mass Balance
3.1.4 Sensors
3.1.4.1 In-line Sensors
3.1.4.2 On-line Sensors
3.1.5 Introduction to the Control Methods of a Wastewater Treatment Plant
by Activated Sludge
3.1.6 Conclusion and Perspectives
Acknowledgement
References
Wastewater Quality Monitoring and Treatment Edited by P. Quevauviller, O. Thomas and A. van der Beken
C
2006 John Wiley & Sons, Ltd. ISBN: 0-471-49929-3
162 Elements of Modelling and Control of Urban Wastewater Treatment Systems
3.1.1 INTRODUCTION
A wastewater treatment plant (WWTP) is an intricate system made of unit operations
based on physical, biological and physico-chemical principles. Its aim is principally
the removal of organic, nitrogen and phosphorus pollution. The basic processes are
complex and the various arrangements of the unit operations which can be proposed
lead to many possible configurations of WWTPs. It is difficult to describe in detail
all of the processes here and only the basics of biological treatment by activated
sludge will be examined. It is the most widespread for WWTPs of medium and
large size. The interested reader will find more details in Henze et al. (Henze et al.,
2000). We focus our attention on the most important parameters for optimization
and process control of pollution removal in large plants, where spatial distribution of
substrate and nutrient in the reacting system plays a large role. In smaller plants, time
scheduling can replace spatial gradients as in sequencing batch reactors for example.
Whatever the case and in spite of the perturbations in terms of flow, composition and
concentration experienced at the inlet of any WWTP, specifications on the discharged
water should be kept within strict limits to avoid taxes and penalties. Different tools
for monitoring and process control are also presented.
3.1.2 SHORT DESCRIPTION OF THE BIOLOGICAL
PROCESS BY ACTIVATED SLUDGE
The biological step (often called secondary treatment) is an essential part of the
WWTP. At the inlet of the plant, the water is usually pretreated to remove gross
debris (grit removal) and can be further treated in a primary settler, which will elim-
inate a large part (usually 40–50 %) of the particulate pollution. In doing so, part of
the biodegradable pollution is indeed removed, which might not always be a good
idea: denitrification, one of the steps involved in nitrogen pollution removal, requires
a certain balance between carbon and nitrogen and an external carbon source is often
added in that step. This could be avoided (or at least limited) by direct injection into
the biological reactor of unsettled wastewater. The principle of activated sludge is the
intensification in a reactor of the principle of self-purification, which is naturally oc-
curring in the environment, in presence of a much higher bacterial concentration than
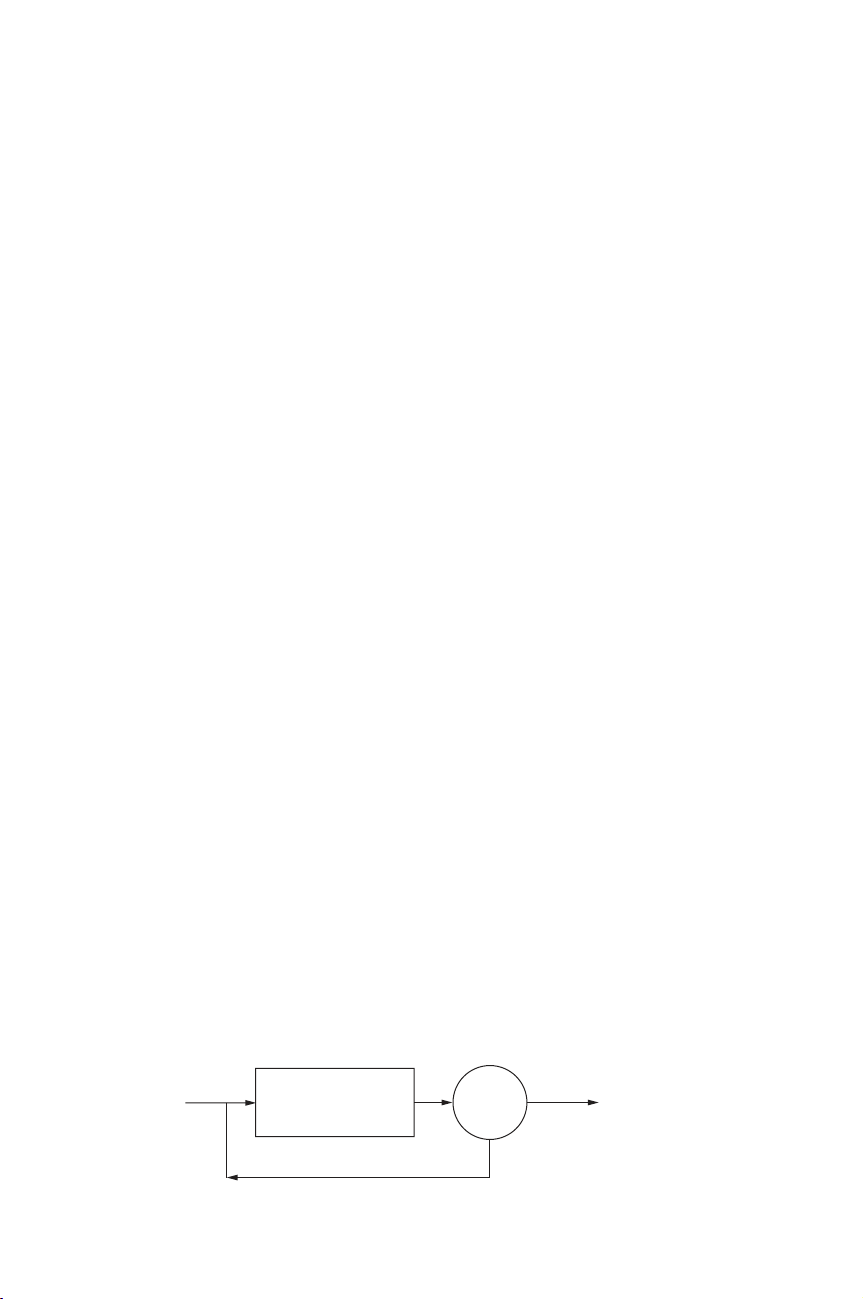
in rivers or lakes. The task of the secondary clarifier (Figure 3.1.1) is to separate the
biological reactor by
activated sludge
return sludge
purified
water
clarifier
Figure 3.1.1 Schematic representation of an activated sludge system
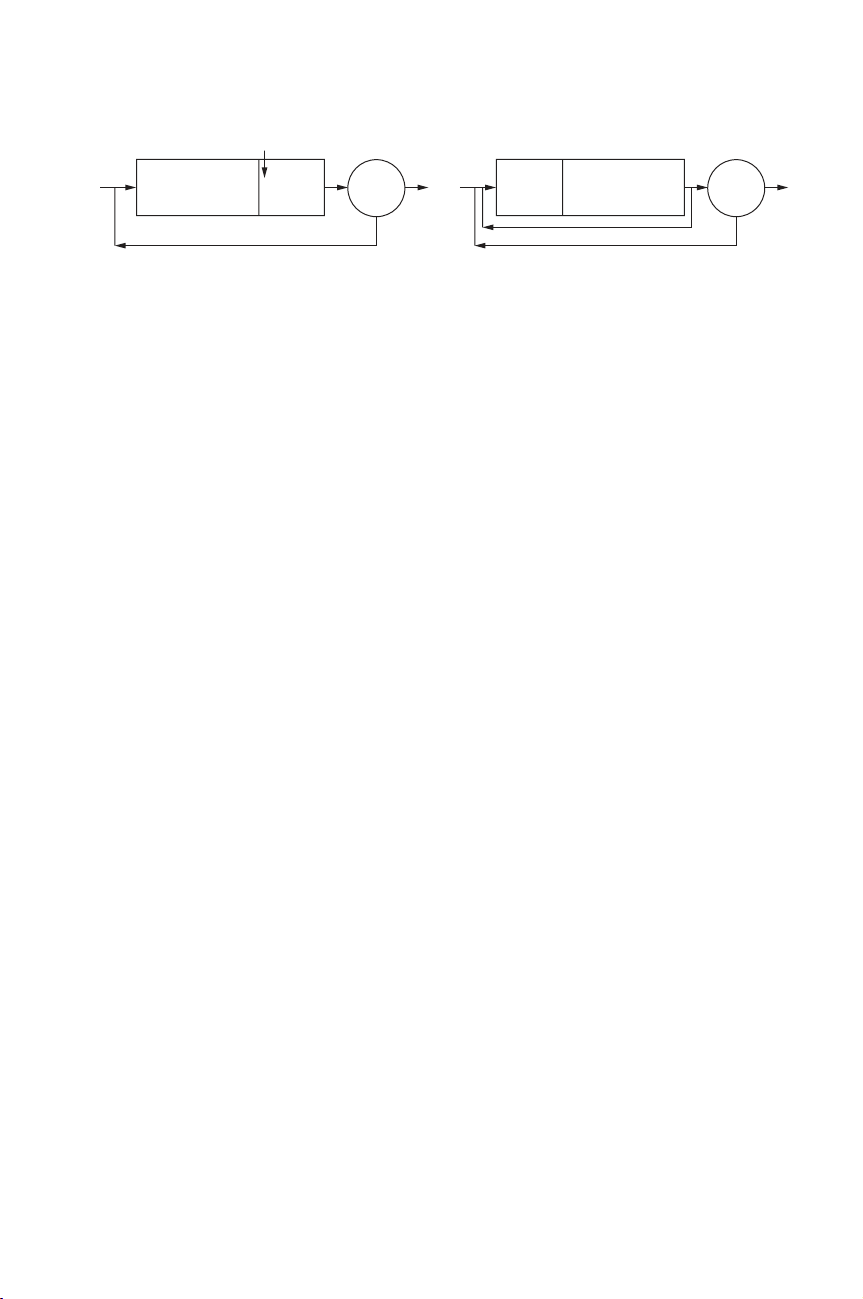
Process Parameters 163
aerobic zone anoxic
zone
Post-denitrification
aerobic zone
anoxic
zone
Pre-denitrification
C
Figure 3.1.2 Schemes of different activated sludge reactors with anoxic zone
flocculated bacteria (sludge flocs) from the treated water. The sludge is returned to
the inlet of the reactor and the purified water is polished in a tertiary stage (post-
treatment of phosphorus, filtration, disinfection, etc.) and/or discharged.
In the presence of oxygen, carbon and a small amount of nitrogen (from ammonia
and hydrolysed organic nitrogen) are metabolized by heterotrophic biomass and most
of the nitrogen by autotrophic bacteria. The latter produced nitrates can be reduced
by heterotrophs under anoxic conditions. As indicated previously, organic matter
is needed for this reaction and therefore an addition of carbon (such as methanol)
is often necessary. In the case of a pre-denitrification system, mixed liquor from
the outlet of the reactor is recycled to the anoxic zone. Some of the most classical
schemes are presented in Figure 3.1.2.
In order to ensure the best process efficiency, different parameters must be known
and controlled: the main reactions of pollution removal and their kinetics; the spatial
distribution of the substrates with respect to the micro-organisms and therefore the
reactor hydrodynamics; the aeration capacity and therefore the oxygen transfer; and
the variability of the wastewater, in terms of composition, concentration and flow rate.
3.1.3 PROCESS PARAMETERS
3.1.3.1 Biokinetics
Many different compounds and micro-organisms are found in a biological wastewater
system. In addition, the ecosystem is never at steady state. Therefore, an exact and
complete kinetic model is out of reach. For many years the scientific community has
tried to provide models of reasonable complexity, able to describe the main steps
of activated sludge behaviour. The basic model is ASM1 (Activated Sludge Model
n◦1), devoted to carbon and nitrogen removal (Henze et al., 1987). Improved versions
have been proposed, such as ASM2, which takes into account phosphorus removal,
and ASM3 (IWA, 2000).
ASM1 is a good compromise between the description of the complex reality of
biological reactions and the simplicity of a model. The identification of any model
parameter should be possible theoretically (structural identifiability) and experimen-
tally through experiments which can be run in the laboratory as well as on full-scale
systems.

![Đề thi Giao tiếp trong kinh doanh học kì 3 năm 2023-2024 có đáp án [kèm đề thi]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250206/gaupanda072/135x160/2841738814667.jpg)



![Phân biệt Many, Some, Few, A Few, Several khác nhau như thế nào? [Giải thích chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2013/20130926/noiaybinhyen123/135x160/3021380166974.jpg)






![Tài liệu Từ vựng tiếng Anh Trung cấp [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250913/nguyentuan250421@gmail.com/135x160/99491757910839.jpg)
![Tài liệu Từ vựng Tiếng Anh theo chủ đề [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250913/namdhuet@gmail.com/135x160/83251757753810.jpg)



![Tài liệu Từ vựng tiếng Anh cho bé [chuẩn nhất/mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250731/huadaithesang2509@gmail.com/135x160/18631754013896.jpg)








