B Y TỘ Ế
C C QU N LÝ D CỤ Ả ƯỢ
-------
C NG HÒA XÃ H I CH NGHĨA VI T NAMỘ Ộ Ủ Ệ
Đc l p - T do - H nh phúc ộ ậ ự ạ
---------------
S : ố5197/QLD-ĐK
V/v công bố danh m c nguyên li u đ ụ ệ ể
s n xu t thu c theo h s đăng ký thu cả ấ ố ồ ơ ố
đã có gi y đăng ký l u hành t i Vi t ấ ư ạ ệ
Nam đc nh p kh u không ph i th c ượ ậ ẩ ả ự
hi n c p phép nh p kh u - Đt 164ệ ấ ậ ẩ ợ
Hà N i, ngày 09 tháng ộ4 năm 2019
Kính g i:ử Các c s đăng ký, s n xu t thu c trong n c.ơ ở ả ấ ố ướ
Căn c Lu t d c s 105/2016/QH13 ngày 06/4/2016;ứ ậ ượ ố
Căn c Ngh đnh 54/2017/NĐ-CP ngày 08/5/2017 quy đnh chi ti t m t s đi u và bi n pháp thi ứ ị ị ị ế ộ ố ề ệ
hành Lu t D c;ậ ượ
Căn c Ngh đnh s 155/2018/NĐ-CP ngày 12/11/2018 s a đi, b sung m t s quy đnh liên ứ ị ị ố ử ổ ổ ộ ố ị
quan đn đi u ki n đu t kinh doanh thu c ph m vi qu n lý nhà n c c a B Y t ;ế ề ệ ầ ư ộ ạ ả ướ ủ ộ ế
C c Qu n lý D c thông báo:ụ ả ượ
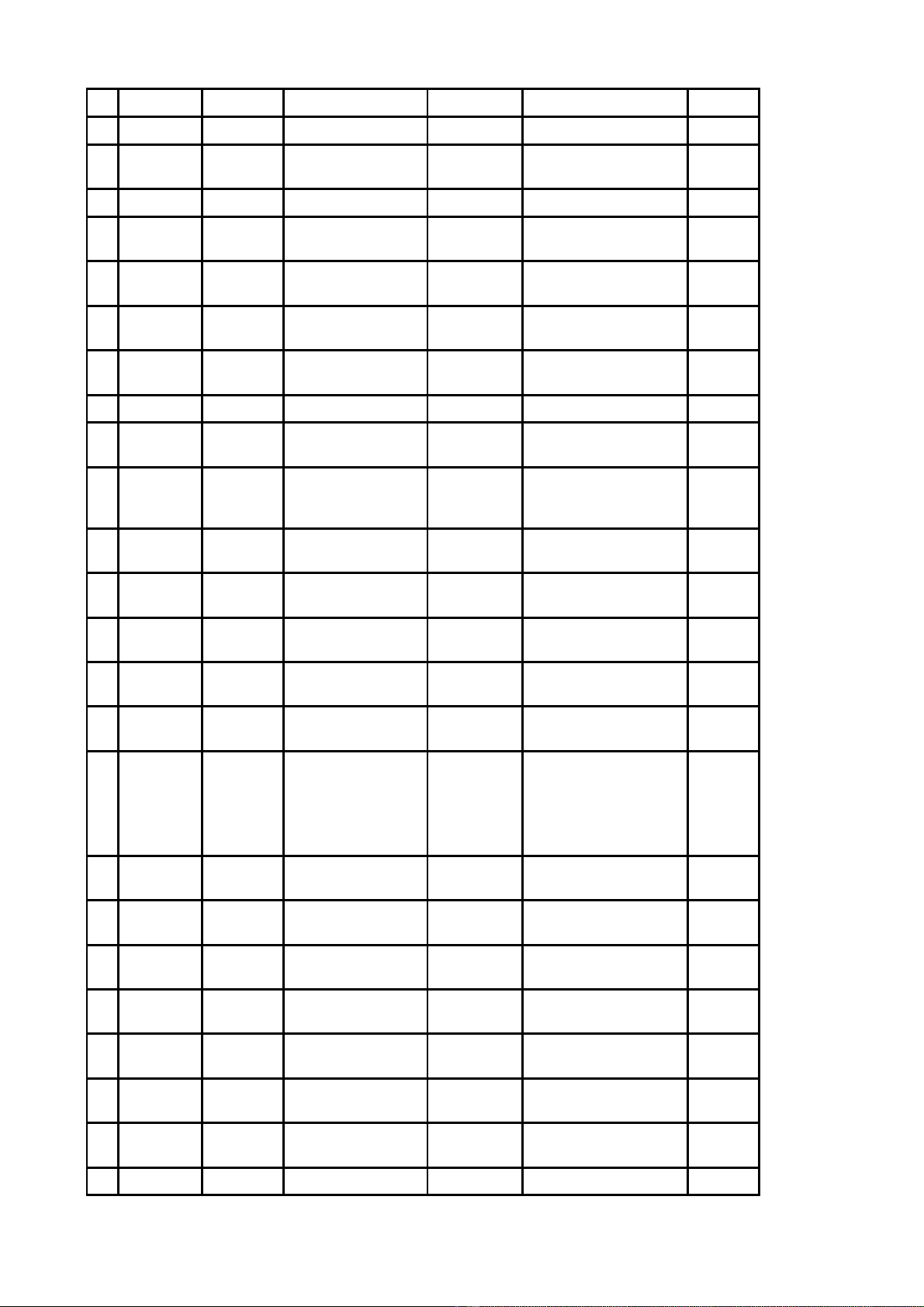
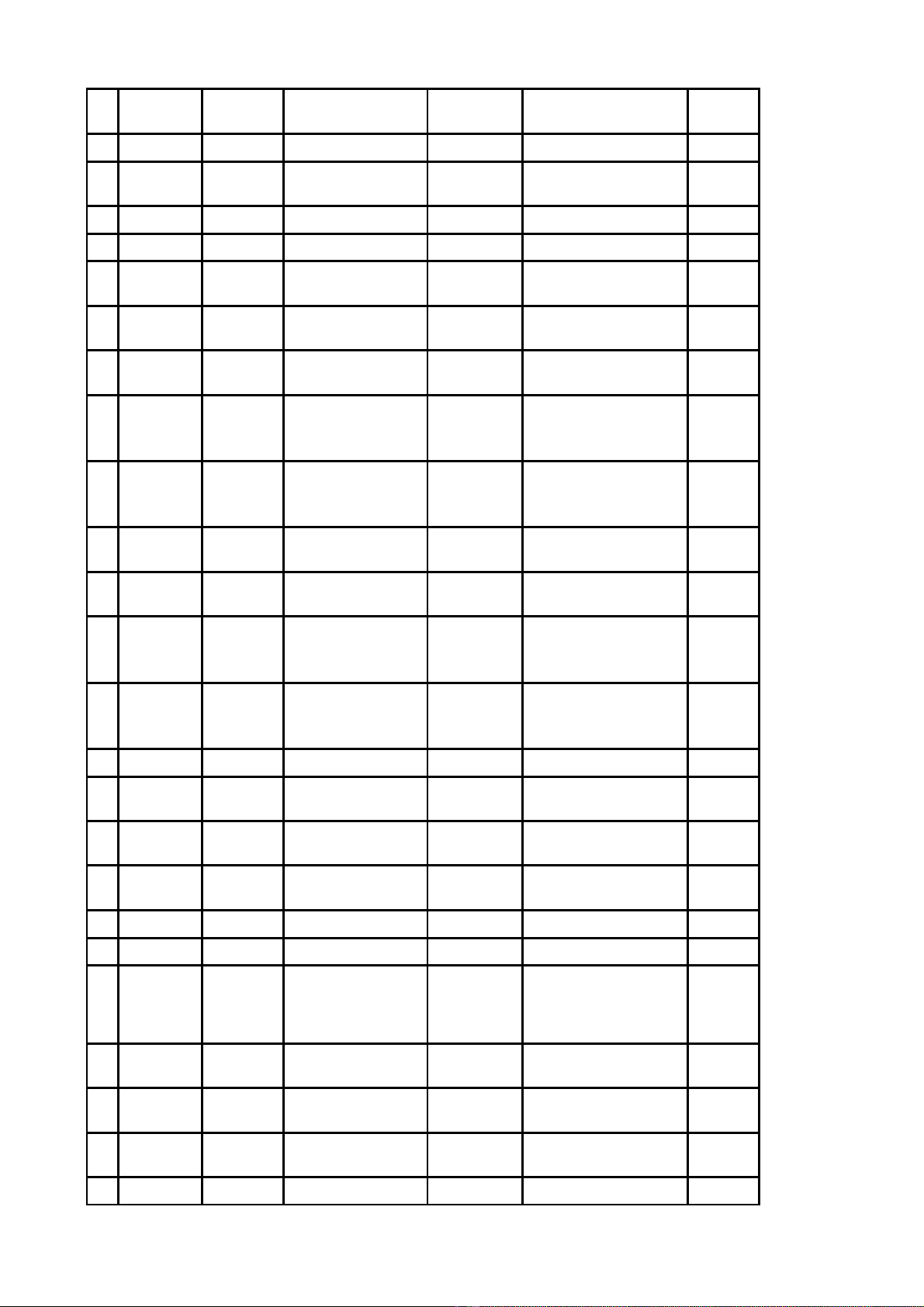
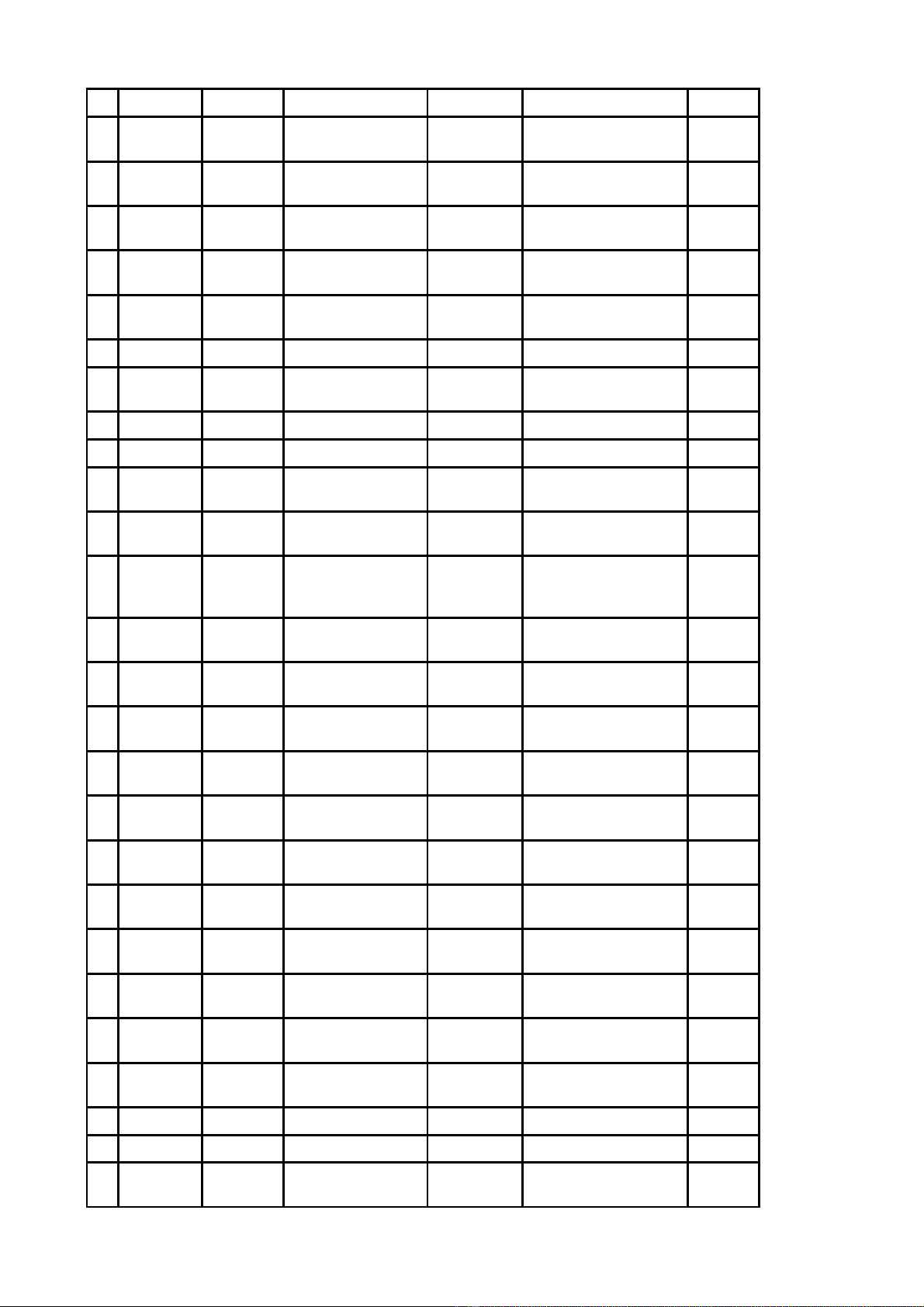
Công b Danh m c nguyên li u đố ụ ệ ểs n xu t thu c theo h s đăng ký thu c đã có gi y đăng ký ả ấ ố ồ ơ ố ấ
l u hành t i Vi t Nam đc nh p kh u không ph i th c hi n c p phép nh p kh u - Đt 164 ư ạ ệ ượ ậ ẩ ả ự ệ ấ ậ ẩ ợ
(theo danh m c đính kèm).ụ
Danh m c nguyên li u đụ ệ ểs n xu t thu c đc công b nêu trên đăng t i trên trang thông tin ả ấ ố ượ ố ả
đi n t c a C c Qu n lý D c t i đa ch : www.dav.gov.vnệ ử ủ ụ ả ượ ạ ị ỉ .
C c Qu n lý D c thông báo đ các công ty s n xu t bi t và th c hi n.ụ ả ượ ể ả ấ ế ự ệ
N i nh n:ơ ậ
- Nh trên;ư
- CT. Vũ Tu n C ng (đ b/c);ấ ườ ể
- T ng C c H i Quan (đ ph i h p);ổ ụ ả ể ố ợ
- Phòng QLKDD;
- Website C c QLD;ụ
- L u: VT, ĐKT (QH).ư
TUQ. C C TR NGỤ ƯỞ
TR NG PHÒNG ĐĂNG KÝ THU CƯỞ Ố
Nguy n Huy Hùngễ
DANH M CỤ
NGUYÊN LI U ĐỆ ỂS N XU T THU C THEO H S ĐĂNG KÝ THU C ĐÃ CÓ GI YẢ Ấ Ố Ồ Ơ Ố Ấ
ĐĂNG KÝ L U HÀNH T I VI T NAM ĐC NH P KH U KHÔNG PH I TH C HI NƯ Ạ Ệ ƯỢ Ậ Ẩ Ả Ự Ệ
C P PHÉP NH P KH U - ĐT 164Ấ Ậ Ẩ Ợ
Đính kèm công văn s 5197ố/QLD-ĐK ngày 09 tháng 4 năm 2019