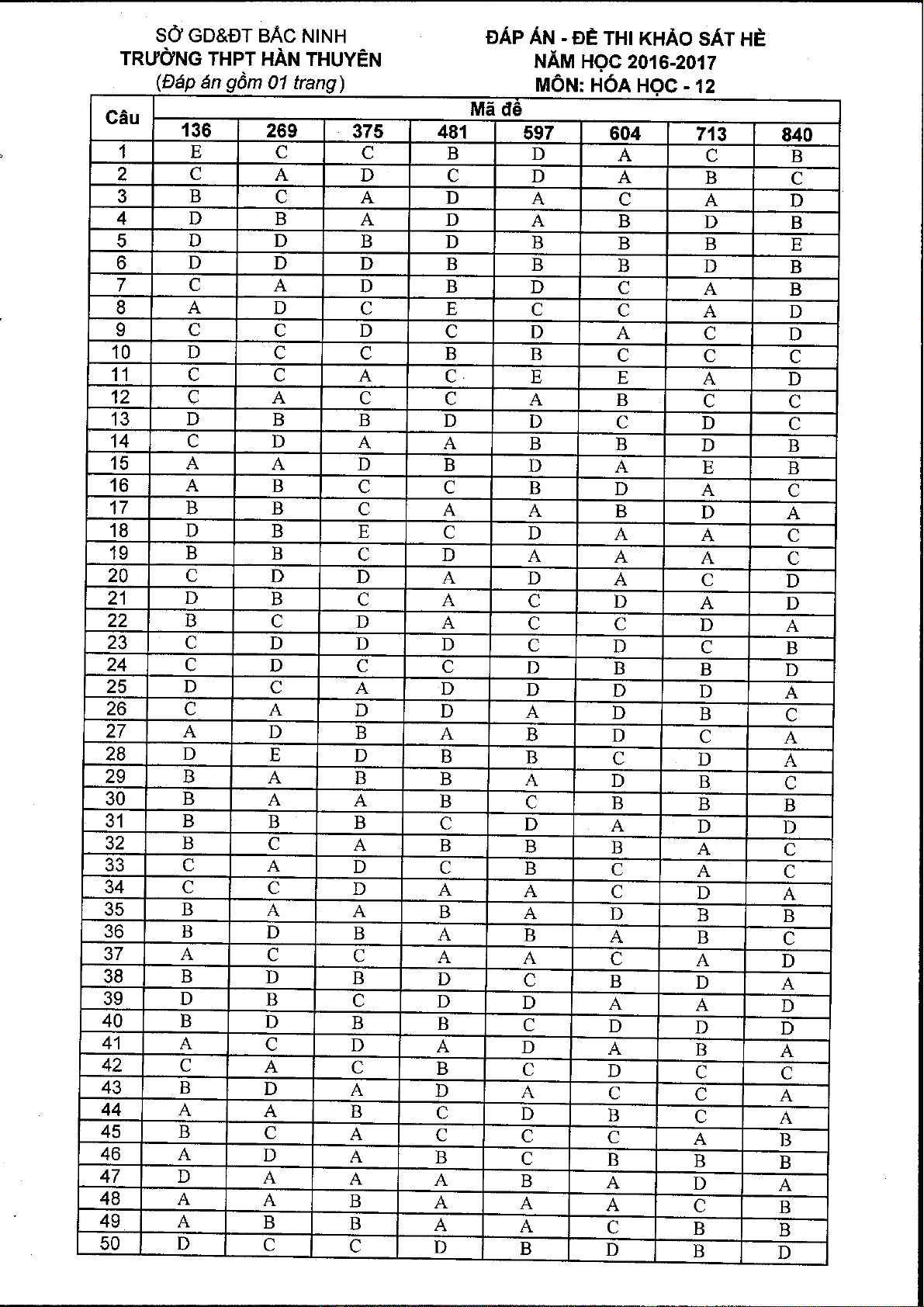
SO GD&DT BAC NINH
'f rudng TFIPT Hin Thuy6n
Dt g6nt 4 trang
EI KIEM TRA CHAT LTIqNG DAU NAM
^NAM HQC 2016-2017
uon : n6.l ugc. ttp: tz
Thiti gian tin hiti: 9t) phtit: khring kd thii gian ptrtir di
Ciu l0: Nhiing mQt tlinh sat sach vdo dung dich Cu(NO:)2. Sau m6t thoi gian l6y dinh sit ra, ldm kh6,
thAy kh6i luong dinh sdt tdng I garn. Kh6i lugng sit tld phan ung ld
A. 3,5 gam. B. 7,0 gam. C. 5,6 gam.
CAu 11: Khi nhiet phan, mu6i nitral ndo sau ddy t4o ra kim loai ?
c.2. D.5.
t. ,
mQt ddn xudl monobrtrm duy nhiit co lr kh6i hoi d6i vcri
B. 2,2-<limetylpropan.
D. 2,8 gam.
D. Fe(NOj)3.
khi dun n6ng ancol c6 cdng lhuc
A. 3-metylbut-l -en. B. 2-rretylbut- 1-en. C. 2-metylbut-2-en. D. 3-metylbur2-en.
Tranp. l14 - \1i de 48 |
Ngdy thi 26108/2016 (50 cdu trdc nghiQm)
llo. tcn thi sinh:.................. ....S0 bao danh:
, .i. ,, i'
Cho biCt kh6i luo. ng nguyen tu (theo dvC) cia c6c nguy€n t6:
Me.rd 4st
tl: l; Li =7:C: l2; N: l4; O: 16; Na:23;Mg:24: Al:27;P = 3l;S:32; Cl:35,5; K:39; Cra
= 40; Cr = 52; Mn = 55: Fe:56; Cu = 64'Zn- 65; Br - 80; Ag = 1081 Ba = 137;Pb=207.
CAu 1: Cho phin ung: Cu + HNO: --- Cu(NO:)z + NO + H:O. Khi h6 s6 cin bang phdn ung ld
nguydn vi ttri gidn thi s6 phan tft FINOr bi khu ld
A.6. 8.2. c.8. D.4.
Cliiu 2: Mdt hidrocacbon X cdng hop voi axit HCI theo ti lQ mol 1:l t4o sin phdm c6 thdnh phdn kh6i
luong clo lit 45,223%. COng thric phdn tri cria X ld
A. C:Hq. B. Czllr. C. C:Ha. D. CrHe.
Cfiu 3: D6t chdy hodn toin m gam h6n hqp 3 anool dcyn chuc, thu6c cirng ddy ddng ddng. thu ducr. c 3,808
lit khi CO2 idktc) vd 5,4 garn ll2O. Gid tri cua nr ld
A. 5,42. B. 7.42. C. 5,72. D. 4,72.
Ciu 4: Oxit kirn loqi bi khir biri khi CO 0 nhiQt d$ cao ld
A. K2O. l]. Al2Oj. C. MgO. D. CuO.
Ciu 5: D6t chdy hodn toirn m6t andehit X, thu duoc s6 mol CO2 bdng s6 mol HuO. N6u cho X t6c dung
voi luong du AgzO (ho4c AgNO3) trong dung dich NFI:, sinh ra s6 mol Ag g6p b6n lAn s6 mol X dl phAn
itng. C6ng thrlc cira X ld
A. CH3CI-IO. B. (CHO)r. c. c2H5cHo. D. HCHO.
CAu 6: 56 ancol ddng phdn cAu tqo cira nhau c6 c6ng thric phdn tir C5H12O. tzic dung vrji CuO dun n6ng
sinh ra xeton li
4.4. ts.3.
CAU 7: Khi brom h6a mQt ankan chi thu duo. c
lrirlrc lir 75.5. T€n cira anl<an d6 lir
A. 2,2,3trime1ylpentan.
A. NaNOr. B. Cu(NO:)2. C. AgNO3.
Cffu 12: T€n gqi cira anken (siur phAm chinh) thu duoc
(CH:)zCHCH(OH)CH3 voi dung dich FIzSO+ ilac ld
C. 3,3-dimetylhecxan. D. isopentan.
Ciu 8: Ancol ndo sau ddy c6 s6 nguy6n tir cacbon bing s6 nh6m -OH?
A. Glixerol B. Etilenglicol C. Ancol benzylic D. Propan- 1,2-diol
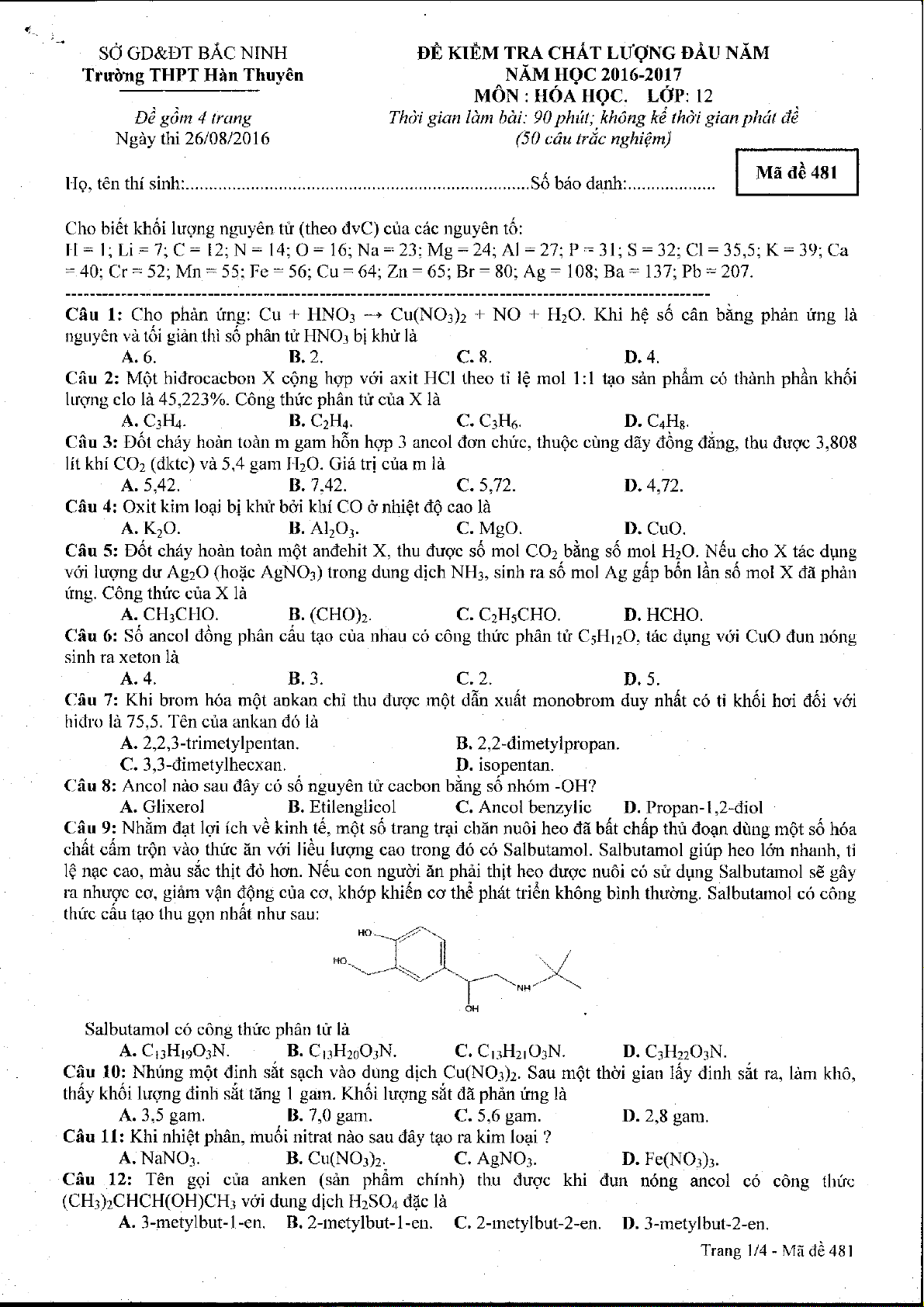
Cff_u 9: Nhim <tat l<vi ich vA kinh.t6, m6t s6 trang trai chdn nuOi heo di bdt chdp thir doqn dr)ng rndt sd h6a
chdt cdm tr6n vdo thfc [n vdi lidu lugrrg cao trong d6 co Salbutamol. Salbutamol girip heo l6n nhanh, 1i
l$ nqc cao, mdu sio thit d6 hon. Ndu con ngudi in phdi thit lreo dugc nudi c6 sri dpng Salbutamol s6 gAy
ra nhuoc co, gi6m v{n dQng cira cn, khdp khi6n co thii ph6t tri6n kh6ng binh thudng. Salbutamol c6 cdng
thtc cdu tao thu gon nhdt nhu sau:
Salbutamol c6 cdng thfc phdn tu ld
A. C13H1eO.rN. B. CrrHzoO:N. C' CrrHzrO:N. D. CIH:2O]N.
I
.1-
| '""
OH