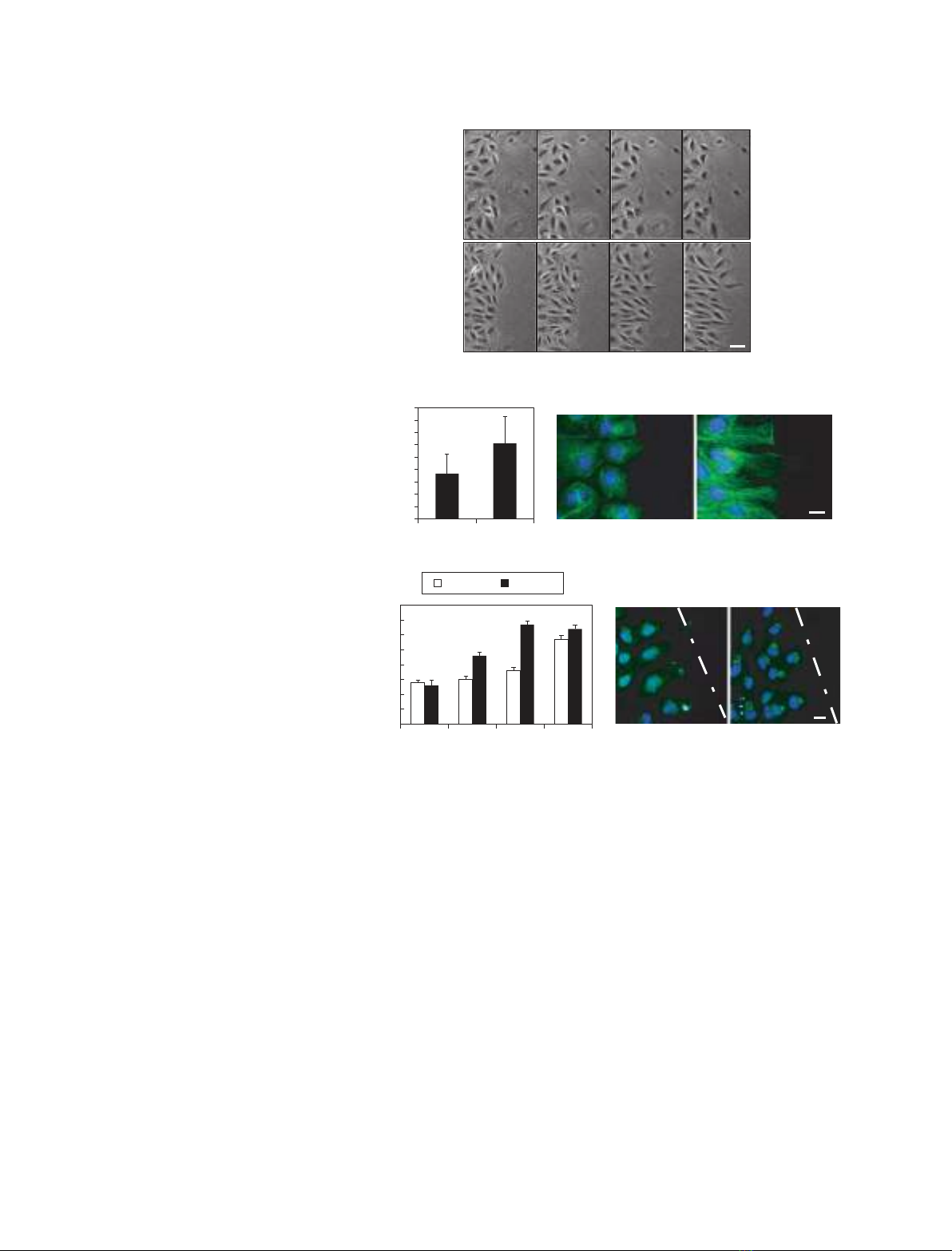
Cas utilizes Nck2 to activate Cdc42 and regulate cell
polarization during cell migration in response to wound
healing
Kohei Funasaka
1
, Satoko Ito
2
, Hitoki Hasegawa
2
, Gary S.Goldberg
3
, Yoshiki Hirooka
1
,
Hidemi Goto
1
, Michinari Hamaguchi
2
and Takeshi Senga
2
1 Department of Gastroenterology, Nagoya University Graduate School of Medicine, Japan
2 Division of Cancer Biology, Nagoya University Graduate School of Medicine, Japan
3 Molecular Biology Department, University of Medicine and Dentistry of New Jersey, Stratford, NJ, USA
Introduction
The establishment of cell polarity is essential for a
variety of cellular functions, such as cell division, dif-
ferentiation and migration; however, the molecular
mechanisms underlying cell polarization have not been
elucidated thoroughly. Genetic and cell biological stud-
ies have identified several molecules that are important
for cell polarity. Among these proteins, Cdc42, a Rho
family GTPase conserved in a wide range of organ-
isms, has been found to play a pivotal role for the
establishment of cell polarity [1–3]. In yeast, Cdc42 is
required for polarized bud formation during cell divi-
sion and morphological changes in response to phero-
mone signaling [4]. In multicellular organisms, cell
polarity is determined by extracellular stimuli, such as
chemoattractant gradients and cell–cell contact. Locali-
zation and activation of Cdc42 in response to these
environmental changes are key events leading to cell
polarization [5,6].
Cas is a multiadaptor protein that regulates various
signaling pathways in response to extracellular stimuli,
including growth factors and integrin-mediated cell
adhesion [7–9]. Cas was originally identified as a
highly phosphorylated protein in cells transformed by
v-Src and v-Crk [10,11]. Cas contains an N-terminal
SH3 domain, proline-rich regions and a substrate
domain with multiple tyrosine phosphorylation sites
Keywords
Cas; Cdc42; Crk; Nck; polarity
Correspondence
T. Senga, Division of Cancer Biology,
Nagoya University Graduate School of
Medicine, 65 Tsurumai-cho, Showa-ku,
Nagoya 466-8550, Japan
Fax: +81 52 744 2464
Tel: +81 52 744 2463
E-mail: tsenga@med.nagoya-u.ac.jp
(Received 14 April 2010, revised 1 June
2010, accepted 28 June 2010)
doi:10.1111/j.1742-4658.2010.07752.x
Integrin-mediated activation of Cdc42 is essential for cell polarization,
whereas the integrin adaptor protein Cas is required for cell migration dur-
ing wound healing. After phosphorylation on tyrosine residues, Cas recruits
the adaptor proteins Crk and Nck to execute integrin-mediated signals.
However, the mechanisms leading to Cdc42 activation and its relationship
with Cas, Crk and Nck have not been elucidated clearly. In the present
study, we demonstrate that Cas utilizes Nck2 to activate Cdc42 and induce
cell polarization in response to wounding. By contrast, Cas recruits CrkII
to activate Rac1 and promote the extension of cell protrusions needed for
cell motility. These results indicate that Cas utilizes Nck2 and CrkII in a
coordinated set of distinct pathways leading to cell migration.
Structured digital abstract
lMINT-7909509:Cas (uniprotkb:Q61140) and Nck2 (uniprotkb:Q8BQ28)colocalize (MI:0403)
by fluorescence microscopy (MI:0416)
Abbreviations
CasKo, homozygous null Cas knockout; CasWt, CasKo transfected with wild-type Cas; DAPI, 4¢,6¢-diamino-2-phenylindole dihydrochloride;
GST, glutathione S-transferase; PAK, p21-activated kinase; PBD, p21 binding domain; PIX, PAK-interacting guanine nucleotide exchange
factor; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-D]pyramidine; siRNA, small interfering siRNA.
3502 FEBS Journal 277 (2010) 3502–3513 ª2010 The Authors Journal compilation ª2010 FEBS