
62 Alternative Methods
biomarkers, whole-organism tests and biological early warning systems for bio-
logical monitoring (Allan et al., 2006). These tools, many being under validation,
even if they are commercially available, are actually designed for water bodies mon-
itoring and very few for wastewater. However, considering their complementary
nature with reference and other alternative methods, there are several new methods
for biological monitoring. Further developments will be devoted to direct applica-
tion to wastewater quality. Meanwhile, a lot of emerging tools can already be used
for discharge toxicity monitoring, such as bioassays and biological early warning
systems (see Chapter 5.1). Other emerging tools designed for chemical monitoring
are passive samplers, immersed in a stream, for the selective adsorption and con-
centration of micropollutants. A recent review (Vrana et al., 2005) has pointed out
the huge development of this approach for water quality monitoring. Even if only
a few applications exist for wastewater quality monitoring with analysis of polar
organic compounds (Alvarez et al., 2005) or trace metals and organic micropollu-
tants (Petty et al., 2004), the use of passive samplers appears to be a very promising
technique, even if the calibration is difficult as it is strongly dependent on the com-
position of water. This is the reason why applications deal with wastewater discharge
impact.
1.4.4 COMPARABILITY OF RESULTS
The purpose of this section is not to give an exhaustive overview of the tools for qual-
ity control and assurance for water quality (the reader will find complete information
in Quevauviller, 2002), but rather to stress a simple procedure to check the compa-
rability of results between a reference method and an alternative one (candidate for
being recognised as an equivalent method).
There exist very few standards for the purpose. The French experimental stan-
dard (AFNOR XP T90-210, 1999) on the evaluation protocol of a physico-chemical
quantitative analysis (for water analysis) regarding a reference method, defines some
principles and tools for the comparability of methods. Considering the complexity
of the problem, this standard is still experimental, and discussions still exist. How-
ever, the principles of this standard have been chosen for the evaluation procedure
for comparing two methods intended for the detection or quantification of the same
target group or species of microorganisms (ISO 17994, 2004). ISO 17994 provides
the mathematical basis for the evaluation of the average relative performance of two
(quantitative) methods against chosen criteria of equivalence. Another international
standard (ISO 11726, 2004) describes procedures for validating alternative (quan-
titative) methods of analysis for coal and coke either directly by comparison with
the relevant international standard method or indirectly by comparison with refer-
ence materials that have been exhaustively analysed using the relevant international
standard method.
Comparability of Results 63
Reference method (x)
Alternative
method (y)Theoretical line
y = x
Experimental line
y = a.x + b
0
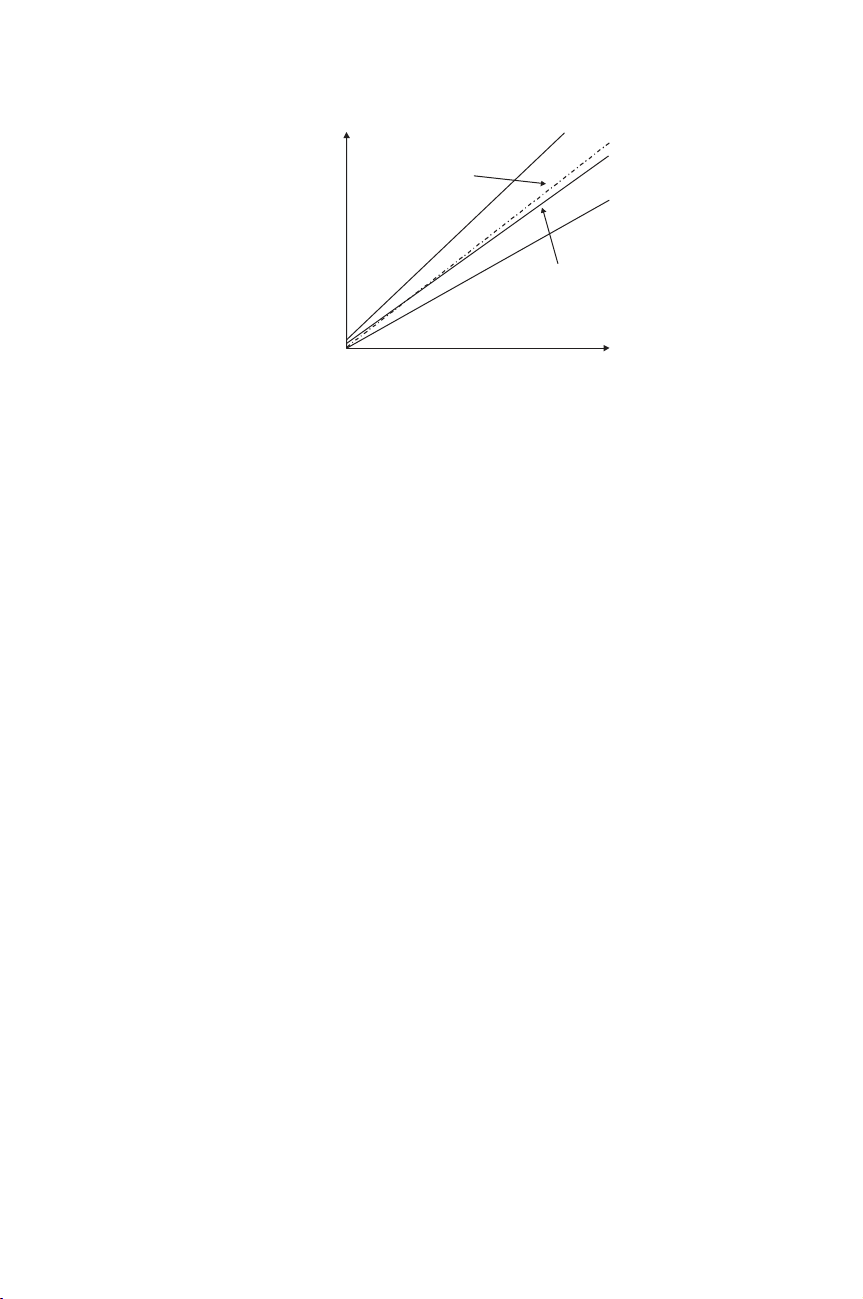
Figure 1.4.2 Comparison between reference and alternative methods
The principles of comparison are simple and schematically based on two steps:
rThe first step aims to calculate the analytical characteristics of the two methods
(reference and alternative), including the reproducibility for a given value (from a
standard solution). A first comparison is carried out on the average values, from a
Fisher–Snedecor test. If the test is conclusive (if the two values are not statistically
different), the second step can be performed.
rThen, the equivalence between methods must be statistically verified by plotting the
results (Figure 1.4.2) and checking the coordinates of the experimental regression
line [comparison of the slope and intercept values which must be not statistically
different from, respectively, 1 and 0, values of the theoretical line (y=x)]. For
the purpose a Student test is carried out.
An example is given in Table 1.4.1, showing the results of the Student test of a compar-
ison from real urban and industrial wastewater (grab samples) for the measurement of
total Kjeldahl nitrogen (TKN). Reference and alternative methods are, respectively,
standard NF EN 25663 and UV/UV procedure (Roig et al., 1999) for TKN. The re-
gression line between the estimated (by the alternative method) and measured values
(by the reference method) is: TKNest =0.96 TKNref +0.86 (R2=0.98). The re-
sults obtained from the comparison of the slope and intercept values to, respectively,
1 and 0, show that the alternative method can be considered as equivalent.
In fact, the scientific decision must be determined by other considerations, such as
the improvement of the alternative method if it brings some consistency advantages
regarding the reference methods (very cheap, rapid, etc.), and the acceptability of
the procedure (Figure 1.4.3).
Once the equivalence between methods is confirmed, the validation procedure
results given for on-/off-line instruments (permanent measurement) must be com-
pleted, taking into account the sampling procedure is different for a laboratory
method and a permanent measurement. For example, considering that regulation
64 Alternative Methods
Table 1.4.1 Results of Student test (95 % confidence interval) for TKN
measurement by UV (method described in Roig et al., 1999)
Student test Values
Slope δ0.9643
Y intercept γ0.8609
Sδ0.02
Sγ0.63
Degree of freedom 55
t0.975 2.01
δ−t0.975*Sδ0.92
δ+t0.975*Sδ1.0045
δ−t0.975*Sδ<1<δ+t0.975*Sδ0.92 <1<1.0045
γ−t0.975*Sγ−0.405
γ+t0.975*Sγ2.127
γ−t0.975*Sγ<0<γ +t0.975*Sγ−0.405 <0<2.127
constraints require 24 h composite sampling before laboratory analysis, the challenge
is to obtain equivalent results with this procedure and with permanent measurement.
In this case, the results to be compared are the mean values for each measurement
during the permanent acquisition, with the reference value of the corresponding
composite sample (Thomas and Pouet, 2005).
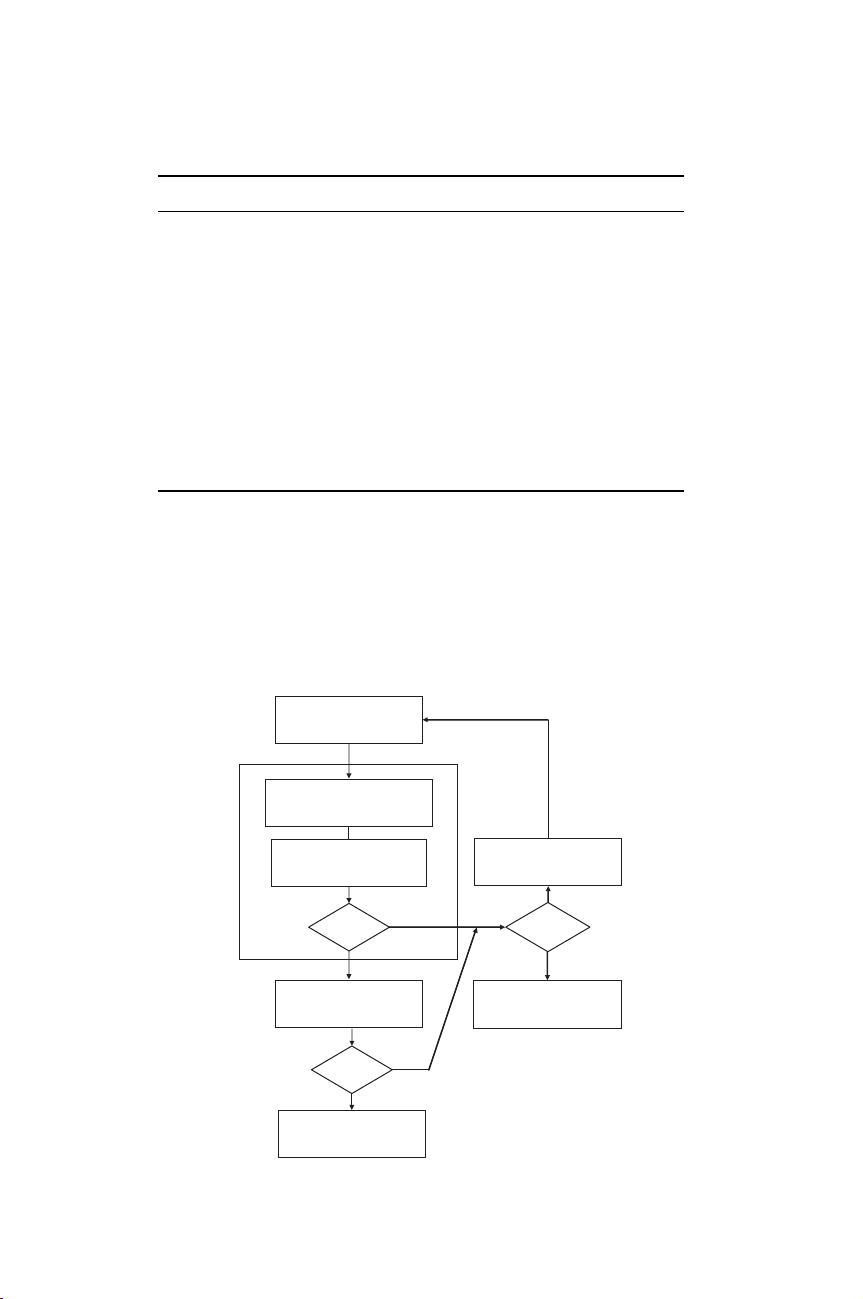
Proposal for
alternative method
Characterisation of standard
and alternative methods
Test of comparability
(reliability)
Proposal for
alternative method
Comparable?
YN
Optimisation
of method
Validation (method)
Abandon
Improvement?
Y
N
Use method
Seek acceptance
Relevance?
Y
N
Validation
Figure 1.4.3 Validation procedure of a candidate alternative (equivalent) method (adapted from
Bruner et al., 1997)

References 65
Finally, the international standards already cited (ISO 17381, 2003; ISO 15839,
2003) should be considered for the general evaluation of ready-to-use test kits
methods and on-line systems. Other procedures can also be cited (Battelle, 2002,
2004), including works in progress in the frame of the European project Swift-WFD
(www.swift-wfd.com).
REFERENCES
AFNOR XP T90-210 (1999) Qualit´e de l’eau – Protocole d’´evaluation d’une m´ethode alternative
d’analyse physico-chimique quantitative par rapport `a une m´ethode de r´ef´erence.
Allan, I.J., Vrana, B., Greenwood, R., Mills, G.A., Roig, B. and Gonzalez, C. (2006) Talanta,69,
302–322.
Alvarez, D.A., Stackelberg, P.E., Petty, J.D., Huckins, J.N., Furlong, E.T., Zaugg, S.D. and Meyer,
M.T. (2005) Chemosphere,61, 610–622.
Battelle (2002) Generic verification protocol for long-term deployment of multiparameter water
quality probes/sondes. http://www.epa.gov/etv/pdfs/vp/01 vp probes.pdf.
Battelle (2004) Generic verification protocol for portable technology for detecting cyanide in water.
http://www.epa.gov/etv/pdfs/vp/01 vp cyanide.pdf.
Baur`es, E. (2002) La mesure non param´etrique, un nouvel outil pour l’´etude des effluents indus-
triels: application aux eaux r´esiduaires d’une raffinerie. PhD thesis, Universit´e Aix Marseille
III, France.
Bonastre, A., Ors, R., Capella, J.V., Fabra, M.J. and Peris, M. (2005) Trends Anal. Chem.,24(2),
128–137.
Bourgeois, W., Burgess, J.E. and Stuetz, R.M. (2001) J. Chem. Technol Biotechnol.,76, 337–348.
Bruner, L.H., Carr, G.J., Curren, R.G. and Chamberlain, M. (1997) Comm. Toxicol., 6, 37–51.
Castillo, L., El Khorassani, H., Trebuchon, P. and Thomas, O. (1999) Water Sci. Technol., 39(10–
11), 17–23.
Dworak, T., Gonzalez, C., Laaser, C. and Interwies, E. (2005) Environ. Sci. Pol., 8, 301–306.
European Commission (1991) Council Directive of 21 May 1991 concerning urban wastewater
treatment (91/271/EEC).
European Commission (2000) Council Directive of 23 October 2000 establishing a framework for
Community action in the field of water policy (2000/60/EC).
Greenwood, R., Roig, B. and Allan, I.J. (2004) Draft report: operational manual, overview of
existing screening methods (available at: http://www.swift-wfd.com).
ISO 5664 (1984) Water quality – Determination of ammonium – Distillation and titration method.
ISO 6778 (1984) Water quality – Determination of ammonium – Potentiometric method.
ISO 7150-1 (1984) Water quality – Determination of ammonium – Part 1: Manual spectrometric
method.
ISO 7150-2 (1986) Water quality – Determination of ammonium – Part 2: Automated spectrometric
method.
ISO 11732 (1997) Water quality – Determination of ammonium nitrogen by flow analysis (CFA
and FIA) and spectrometric detection.
ISO 11348-3 (1998) Water quality – Determination of the inhibitory effect of water samples on the
light emission of Vibrio fischeri (Luminescent bacteria test) – Part 3: Method using freeze-dried
bacteria.
ISO 15839 (2003) Water quality – On line sensors/analysing equipment for water: specifications
and performance tests.

66 Alternative Methods
ISO 17381 (2003) Water quality – Selection and application of ready-to-use test kit methods in
water analysis.
ISO 11726 (2004) Solid mineral fuels – Guidelines for the validation of alternative methods of
analysis.
ISO 17994 (2004) Water quality – Criteria for establishing equivalence between microbiological
methods.
Muret, C., Pouet, M.F., Touraud, E. and Thomas, O. (2000) Water Sci. Technol.,42(5–6), 47–52.
Oliveira-Esquerre, K.P., Seborg, D.E., Bruns, R.E. and Mori, M. (2004a) Chem. Engin. J., 104,
73–81.
Oliveira-Esquerre, K.P., Seborg, D.E., Mori, M. and Bruns, R.E. (2004b) Chem. Engin. J., 105,
61–69.
Petty, J.D., Huckins, J.N., Alvarez, D.A., Brumbaugh, W.G., Cranor, W.L., Gale, R.W., Rastall,
A.C., Jones-Lepp, T.L., Leiker, T.J, Rostad, C.E. and Furlong, E.T. (2004) Chemosphere,54,
695–705.
Quevauviller, Ph. (2002) Quality Assurance for Water Analysis. Water Quality Measurements
Series. John Wiley & Sons Ltd, Chichester.
Roig, B., Gonzalez, C. and Thomas, O. (1999) Anal. Chim. Acta,389, 267–274.
Sperandio, M. and Queinnec, I. (2004) Water Sci. Technol., 49(1), 31–38.
Thomas, O. (1995) M´etrologie des eaux r´esiduaires. Tec et Doc: Paris; Cebedoc: Li`ege.
Thomas, O. and Constant, D. (2004) Water Sci. Technol., 49(1), 1–8.
Thomas, O. and Pouet, M.-F. (2005) Wastewater quality monitoring: on-line/on-site measurement.
In: The Handbook of Environmental Chemistry, 5, part O, Barcelo, D., (Ed.). Springer-Verlag:
Berlin, pp. 245–272.
Thomas, O., El Khorassani, H., Touraud, E. and Bitar, H. (1999) Talanta,50, 743–749.
Vanrollegem, P.A. and Lee, D.S. (2003) Water Sci. Technol., 47(2), 1–34.
Vrana, B., Mills, G.A., Allan, I.J., Dominiak, E., Svensson, K., Knutsson, J., Morrison, G. and
Greenwood, R. (2005) Trends Anal. Chem., 24(10), 845–868.




![Phân biệt Many, Some, Few, A Few, Several khác nhau như thế nào? [Giải thích chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2013/20130926/noiaybinhyen123/135x160/3021380166974.jpg)









![Từ vựng tiếng Anh về thức ăn và giảm cân [mới nhất, đầy đủ]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251217/nglinh.diamond@gmail.com/135x160/53091766028543.jpg)


![Tài liệu Từ vựng tiếng Anh Trung cấp [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250913/nguyentuan250421@gmail.com/135x160/99491757910839.jpg)
![Tài liệu Từ vựng Tiếng Anh theo chủ đề [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250913/namdhuet@gmail.com/135x160/83251757753810.jpg)



![Tài liệu Từ vựng tiếng Anh cho bé [chuẩn nhất/mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250731/huadaithesang2509@gmail.com/135x160/18631754013896.jpg)



