Exploring the role of a glycine cluster in cold adaptation
of an alkaline phosphatase
Konstantinos Mavromatis
1,
*, Iason Tsigos
2,
*, Maria Tzanodaskalaki
2
, Michael Kokkinidis
1,3
and Vassilis Bouriotis
1,2
1
Department of Biology, Division of Applied Biology and Biotechnology, University of Crete, Greece;
2
Institute of Molecular Biology
and Biotechnology (IMBB), Enzyme Technology Division, and the
3
Institute of Molecular Biology and Biotechnology,
Crystallography Division, Heraklion, Crete, Greece
In an effort to explore the role of glycine clusters on the cold
adaptation of enzymes, we designed point mutations aiming
to alter the distribution of glycine residues close to the active
site of the psychrophilic alkaline phosphatase from the
Antarctic strain TAB5. The mutagenesis targets were
residues Gly261 and Gly262. The replacement of Gly262 by
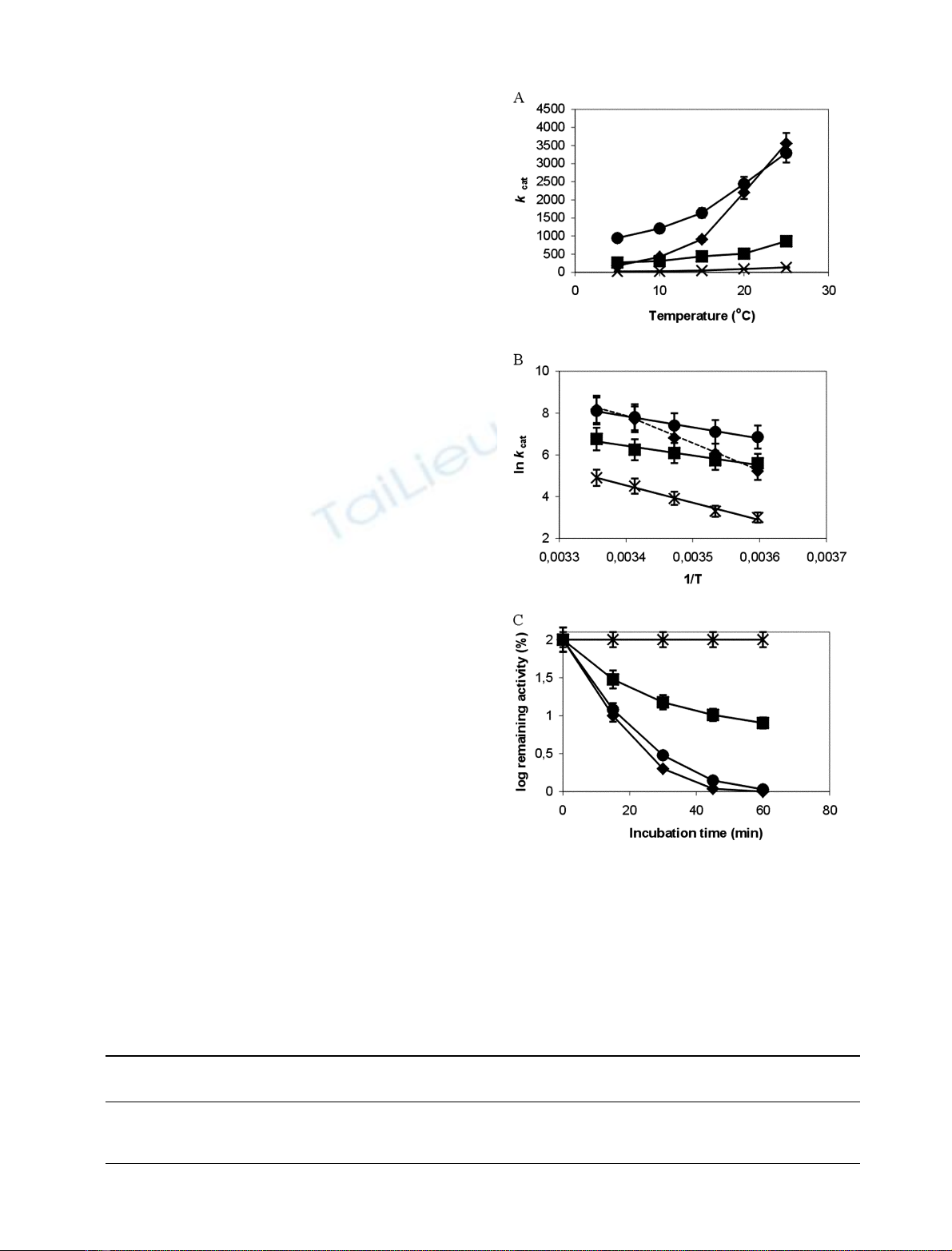
Ala resulted in an inactive enzyme. Substitution of Gly261
by Ala resulted to an enzyme with lower stability and
increased energy of activation. The double mutant G261A/
Y269A designed on the basis of side-chain packing criteria
from a modelled structure of the enzyme resulted in restor-
ation of the energy of activation to the levels of the native
enzyme and in an increased stability compared to the mutant
G261A. It seems therefore, that the Gly cluster in combi-
nation with its structural environment plays a significant role
in the cold adaptation of the enzyme.
Keywords: alkaline phosphatase; psychrophiles; cold
adaptation; structural flexibility; glycine clusters.
Cold adapted enzymes, produced by organisms living in
permanently cold environments, exhibit a higher specific
activity at low temperatures [1–3]. Moreover, this high
catalytic efficiency is consistently accompanied by a lower
thermal stability, although these properties are not always
correlated as shown by recent data from directed evolution
experiments which support the interdependence of these
properties [4–8].
The adaptation to cold is achieved through a decrease in
the activation energy, which results from an increased
protein flexibility, either of the whole protein or of a specific
domain in some multidomain proteins. Furthermore,
evidence from the notothenioid A4-lactate dehydrogenases
support a cold adaptation model in which structural
flexibility increases are confined to small areas of the
molecule, thereby affecting the mobility of adjacent active
site structures and resulting in reduced energy barriers [9].
Therefore, psychrophilic adaptation seems to be associated
with localized rather than global increases in conformational
flexibility [10]. This is in agreement with structural data,
which reveal that only minor modifications are necessary to
convert a mesophilic or thermophilic enzyme into a cold
adapted one [11–14].
Although the strategy of adaptation is unique to each
enzyme [15], it has been observed that amino-acid residues
involved in the catalytic mechanism are generally conserved
in psychrophilic and mesophilic enzymes [1]. This suggests
that generally the molecular basis of cold adaptation is
associated with sequence changes outside the active site.
However, recent work from our group indicated that the
psychrophilic character of an enzyme could also be altered
or masked by mutating active site residues [16]. Several
sequence patterns have been associated with psychrophilic
adaptations, such as decreased levels of Pro and Arg
residues, weakening of intramolecular interactions,
increased solvent interactions, decreased charged residues
interactions, and disulfide bonds [1,2,17]. Increased levels of
Gly residues or the establishment of Gly clusters have been
frequently suggested to be associated with psychrophilicity
[2]. This could be a result of increased local structural
flexibility due to the intrinsic flexibility of Gly residues [18].
However, recent studies of Gly clusters [19] appear to
contradict this assumption. It seems that the correlation
between the occurrence of Gly residues and the stability of
proteins is complex as several parameters from the whole
protein structure are involved and not just the intrinsic
flexibility of Gly residues [20].
We have recently reported the cloning, sequencing and
overexpression of the gene encoding alkaline phosphatase
from the Antarctic strain TAB5 [16]. Based on the crystal
structure (at 2.4 A
˚)ofanEscherichia coli alkaline phospha-
tase variant with a 28% amino-acid sequence identity to the
psychrophilic enzyme, a three-dimensional model of the
psychrophilic enzyme was constructed [21]. We have also
presented mutagenesis data that substantiate the role of the
local flexibility on the psychrophilic character, and catalytic
properties of the enzyme [16]. In the case of alkaline phos-
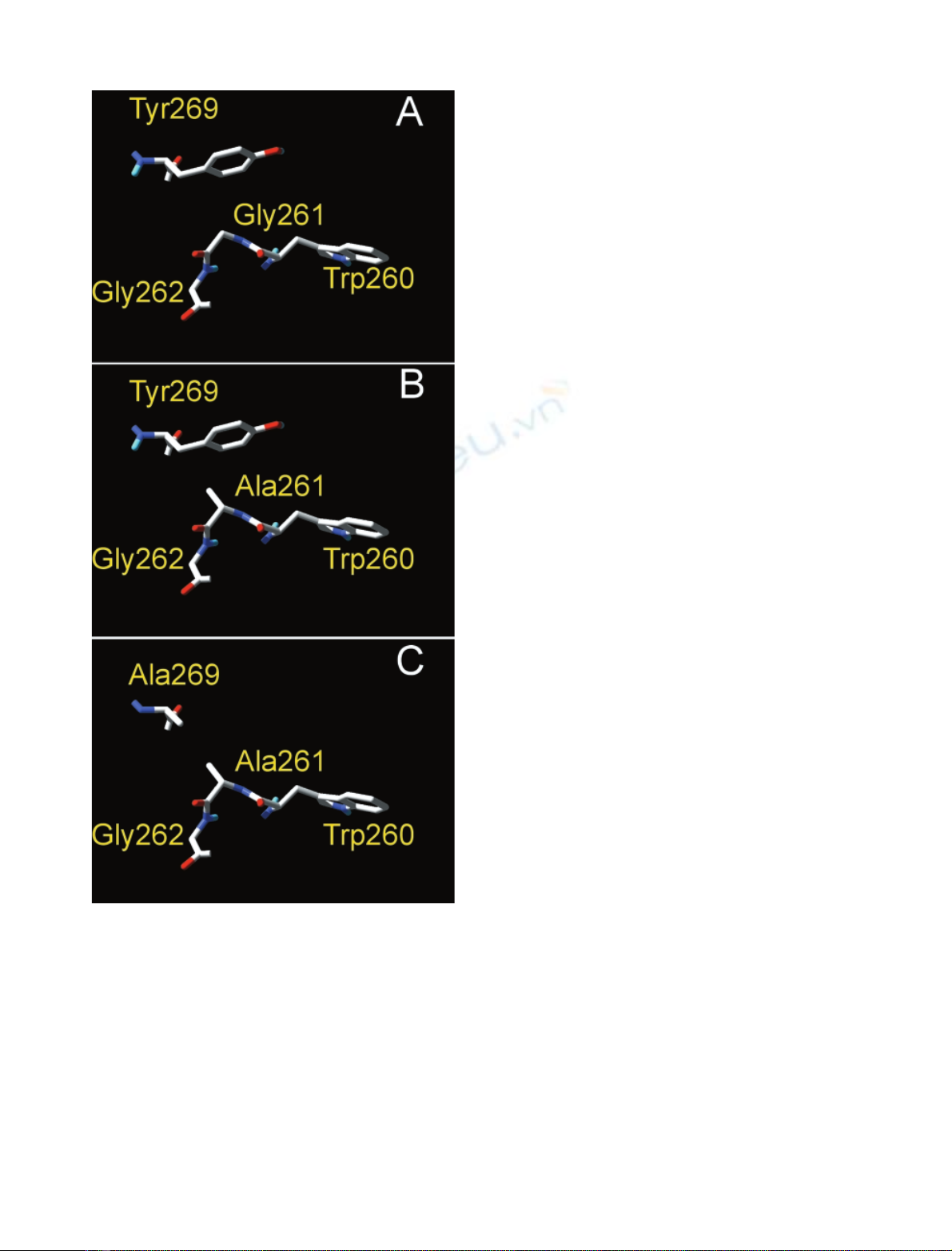
phatases, positions 261, 262 (in TAB5 alkaline phosphatase
numbering) are often occupied by one Gly; this site is next
to one of the catalytic residues (Trp260 in the case of TAB5
alkaline phosphatase). In E.coliand some Bacillus sp., there
Correspondence to V. Bouriotis, Department of Biology,
Division of applied Biology and Biotechnology, University of Crete,
PO Box 1470, Heraklion 711 10, Crete, Greece.
Fax/Tel.: + 30 810 394375, E-mail: bouriotis@imbb.forth.gr
Abbreviation:pNPP, p-nitrophenyl phosphate.
Enzyme: alkaline phosphatase (EC 3.1.3.1).
*Note: these authors have equally contributed to this work.
(Received 12 December 2001, revised 14 March 2002,
accepted 18 March 2002)
Eur. J. Biochem. 269, 2330–2335 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.02895.x