
Inhibitory properties of cystatin F and its localization
in U937 promonocyte cells
Tomaz
ˇLangerholc
1
, Valentina Zavas
ˇnik-Bergant
1
, Boris Turk
1
, Vito Turk
1
, Magnus Abrahamson
2
and Janko Kos
3
1 Department of Biochemistry and Molecular Biology, Joz
ˇef Stefan Institute, Ljubljana, Slovenia
2 Department of Clinical Chemistry, Institute of Laboratory Medicine, University of Lund, Sweden
3 Faculty of Pharmacy, Department of Pharmaceutical Biology, University of Ljubljana, Slovenia
Human papain-like cathepsins were long believed to be
responsible for terminal protein degradation in the
lysosomes. This view changed dramatically when they
were found to be involved in a number of important
cellular processes, such as antigen presentation [1],
bone resorption [2], apoptosis [3] and protein process-
ing [4], as well as several pathologies such as cancer
progression [5], inflammation [6] and neurodegenera-
tion [7]. Their high proteolytic potential, which can be
very harmful, requires the activity of papain-like cath-
epsins to be strictly regulated. Their endogenous pro-
tein inhibitors act as one of the main means of
regulation [8]. The best characterized are the cystatins,
which comprise a superfamily of evolutionarily related
proteins, each consisting of at least one domain of
100–120 amino acid residues with conserved sequence
motifs [8–11]. Type I cystatins (the stefins), stefins A
and B, are cytosolic, 100 amino acid residue-long
proteins lacking disulfide bridges. Type II cystatins,
cystatins C, D, E ⁄M, F, S, SA, SN are longer extra-
cellular proteins, consisting of 120 amino acid resi-
dues and containing two disulfide bridges. Type III
cystatins, the kininogens, are large multifunctional
plasma proteins, containing three type II cystatin-like
domains.
Cystatin F was discovered recently by three inde-
pendent groups. Two of them identified it by cDNA
cloning and named the new inhibitor leukocystatin [12]
Keywords
cathepsin; cysteine protease; inhibition;
cystatin; antigen presentation
Correspondence
T. Langerholc, Department of Biochemistry
and Molecular Biology, Joz
ˇef Stefan
Institute, Ljubljana, Slovenia
Fax: +386 14773984
Tel: +386 14773573
E-mail: tomaz.langerholc@ijs.si
(Received 9 November 2004, revised 31
January 2005, accepted 2 February 2005)
doi:10.1111/j.1742-4658.2005.04594.x
Cystatin F is a recently discovered type II cystatin expressed almost exclu-
sively in immune cells. It is present intracellularly in lysosome-like vesicles,
which suggests a potential role in regulating papain-like cathepsins involved
in antigen presentation. Therefore, interactions of cystatin F with several
of its potential targets, cathepsins F, K, V, S, H, X and C, were studied
in vitro. Cystatin F tightly inhibited cathepsins F, K and V with K
i
values
ranging from 0.17 nmto 0.35 nm, whereas cathepsins S and H were inhib-
ited with 100-fold lower affinities (K
i
30 nm). The exopeptidases, cathep-
sins C and X were not inhibited by cystatin F. In order to investigate the
biological significance of the inhibition data, the intracellular localization
of cystatin F and its potential targets, cathepsins B, H, L, S, C and K,
were studied by confocal microscopy in U937 promonocyte cells. Although
vesicular staining was observed for all the enzymes, only cathepsins H and
X were found to be colocalized with the inhibitor. This suggests that cysta-
tin F in U937 cells may function as a regulatory inhibitor of proteolytic
activity of cathepsin H or, more likely, as a protection against cathepsins
misdirected to specific cystatin F containing endosomal ⁄lysosomal vesicles.
The finding that cystatin F was not colocalized with cystatin C suggests
distinct functions for these two cysteine protease inhibitors in U937 cells.
Abbreviations
mAb, monoclonal antibody; pAb, polyclonal antibody; M6P, mannose-6-phosphate.
FEBS Journal 272 (2005) 1535–1545 ª2005 FEBS 1535
and cystatin F [13]. The third group found overex-
pressed mRNA encoding cystatin F in liver metastatic
tumors and named it cystatin-like metastasis-associated
protein (CMAP) [14]. Cystatin F is an unusual type II
cystatin showing little sequence identity (29–34%) to
other members of the family. Together with cystatin
E⁄M it is the only known human glycosylated type II
cystatin. In addition to two disulfide bonds, common
to all type II cystatins, cystatin F contains two addi-
tional cysteines in positions 1 and 37 (cystatin C num-
bering), which were suggested to form an additional
disulfide bond [13]. Cystatin F has been shown to inhi-
bit cathepsin L (EC 3.4.22.15), papain (EC 3.4.22.2)
and legumain (EC 3.4.22.34; K
i
¼0.3–10 nm), but not
cathepsin B (EC 3.4.22.1), which was therefore sugges-
ted not to be a physiological target of cystatin F
[13,15].
Given that its expression is restricted to hematopoi-
etic cells [12,13] it is likely that cystatin F is involved
in processes of the immune response. Immunomodula-
tory properties have been demonstrated for another
type II cystatin, cystatin C. The process of dendritic
cell maturation leads to a reduced level and distinct
intracellular distribution of cystatin C, favoring the
activity of cathepsin S and hence efficient Ii chain clea-
vage [16]. In contrast, cystatin F mRNA levels are sig-
nificantly upregulated during dendritic cell maturation
[17]. Immunocytochemical staining of cystatin F in
human promonocyte U937 cells displays a vesicular
pattern [18]. In subcellular fractionation experiments
cystatin F coeluted with the peak of b-hexosaminidase
activity, an enzyme typically located in lysosome-like
organelles. Independently, Journet et al. [19] detected
cystatin F as a soluble protein after affinity puri-
fication of mannose-6-phosphate (M6P) containing
proteins. This means that M6P was present in the
N-linked carbohydrate moiety in cystatin F or, alter-
natively, that cystatin F was in complex with another
M6P containing protein. Nevertheless, despite secre-
tion of cystatin F from U937 cells, a high proportion
seems to reside intracellularly in lysosomes or lyso-
some-like organelles [18].
The aim of our study was to identify potential tar-
gets of cystatin F among endogenous lysosomal cys-
teine proteases. First we found that dimers of cystatin
F are inactive as inhibitors of cysteine proteases and
that the monomeric form has to be restored for the
inhibitory potential. After activation of cystatin F we
have studied the in vitro kinetics of the interaction
between cystatin F and several cathepsins, as well
as their intracellular localization in promonocyte
U937 cells, using specific antibodies and confocal
microscopy.
Results
Activation of cystatin F
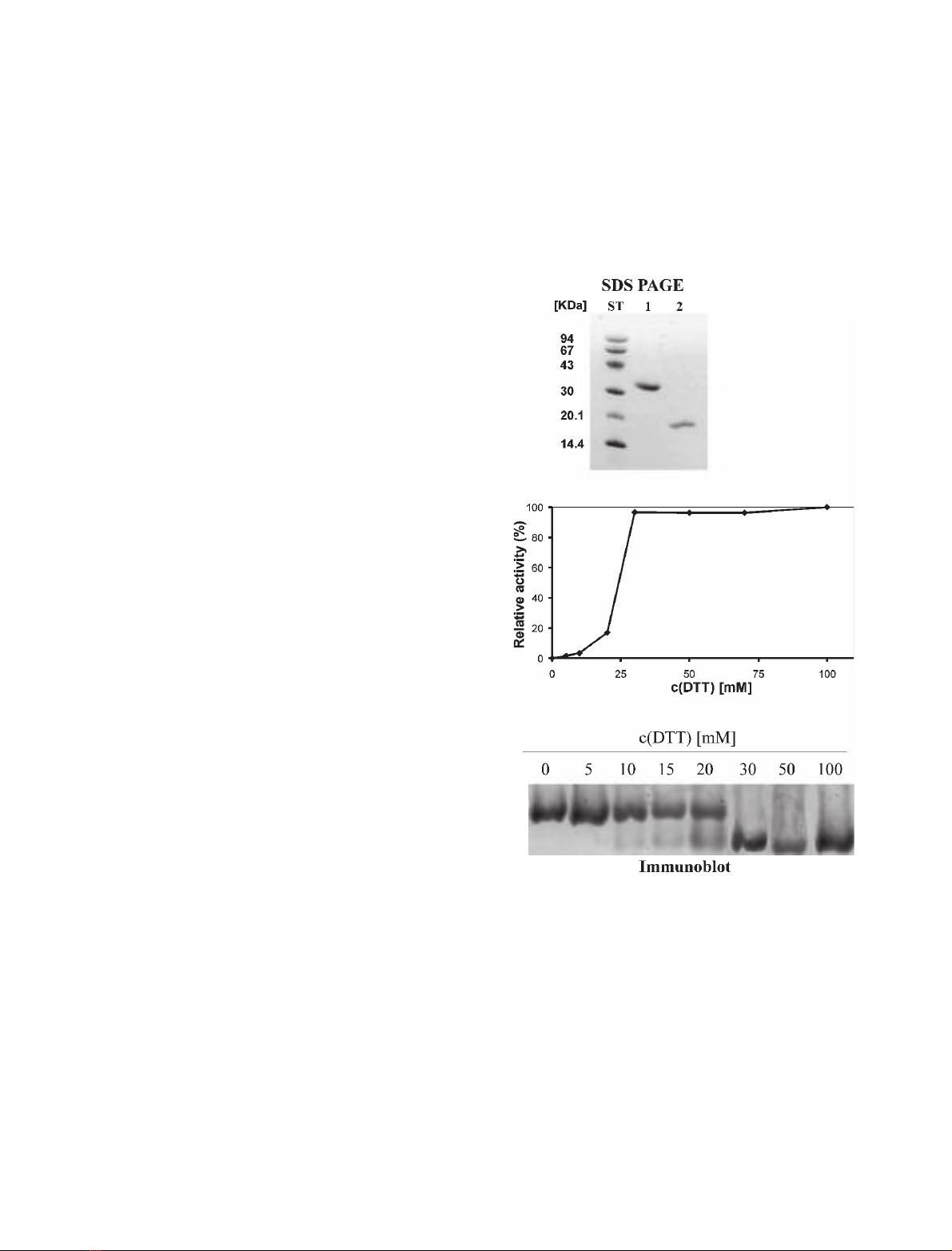
Recombinant cystatin F showed one band on
SDS ⁄PAGE (Fig. 1A) at 17 kDa under reducing con-
A
B
C
Fig. 1. (A) SDS ⁄PAGE of cystatin F; lane 1, no reduction; lane 2,
reduction with 100 mMdithiotreitol; ST, molecular weight stand-
ards. (B) Inhibitory activity of cystatin F against papain. Cystatin F
(500 nM) was incubated 15 min at 37 C in phosphate buffer
pH 6.0 with different concentrations of dithiotreitol. After dilution,
cystatin F was equilibrated with a twofold molar excess of papain.
The residual activity of papain was measured with Z-FR-AMC. Rel-
ative activity of cystatin F is shown, from 0% (uninhibited enzyme)
to 100% (the lowest activity of the enzyme). Dimerization of cysta-
tin F leads to a loss of inhibitory activity. (C) Immunoblot of cystatin
F run on nondenaturing PAGE. Cystatin F (200 nM) was incubated
15 min at 37 C in phosphate buffer pH 6.0 using different concen-
trations of dithiotreitol. Samples were immunoblotted using anti-
human cystatin F polyclonal antibody.
Cystatin F in U937 cells T. Langerholc et al.
1536 FEBS Journal 272 (2005) 1535–1545 ª2005 FEBS

ditions and at 35 kDa under nonreducing conditions.
The latter corresponds to a dimer of cystatin F.
In the absence of reducing agent dithiotreitol, dimeric
cystatin F did not inhibit papain, but its relative activity
(0% for uninhibited enzyme, 100% for the highest inhi-
bition) increased substantially on incubation with 20
and 30 mmdithiotreitol (20 and 100%, respect-
ively). No further changes in relative activity of cystatin
F were observed at dithiotreitol concentrations above
30 mm(Fig. 1B). In addition, the effect of increasing the
concentration of reductant on cystatin F was followed
by electrophoresis under native conditions, where the
transition between 20 and 30 mmdithiotreitol was
accompanied by a shift to a smaller molecular mass
(Fig. 1C). These results suggest that dimerization of
cystatin F is linked to disulfide bond formation, which is
responsible for the loss of inhibitory activity of the pro-
tein. We also noticed that monomerization of cystatin F
was enhanced in acidic environment, especially below
pH 5 (T. Langerholc, unpublished data).
Cystatin F was remarkably stable under reducing
conditions, as no loss of inhibitory activity was
observed even after prolonged incubation at dithiotrei-
tol concentrations as high as 100 mm, and the K
i
value
for the inhibition of papain (K
i
¼1.4 nm) was similar
to that reported for inhibition at low dithiotreitol con-
centration (K
i
¼1.1 nm) [13].
Based on these results, 100 mmdithiotreitol was
used in the cystatin F activation buffer in order to
ensure total conversion of cystatin F to the active
monomeric state prior to kinetic studies. It should be
noted that, after dilution of cystatin F solution to the
final dithiotreitol concentration of 2.5 mm, which was
used in all subsequent inhibition studies, no dimer for-
mation was observed.
Inhibition of potential target enzymes
in U937 cells
In preliminary experiments, nanomolar to submicro-
molar concentrations of cystatin F were found to be
sufficient to completely abolish the activity of cathep-
sins F (EC 3.4.22.41), K (EC 3.4.22.38), L, V (EC
3.4.22.43), S (EC 3.4.22.27) and H (EC 3.4.22.16).
However, the true exopeptidases cathepsins C
(EC 3.4.14.1) and X (EC 3.4.22.-) were not inhibited
at all, even at the highest concentration of the inhib-
itor (200 nmfor cathepsin C and 600 nmfor cathep-
sin X, respectively). Therefore, detailed kinetic studies
were performed only with cathepsins F, K, L, V, S
and H.
A linear dependence of the pseudo first order rate
constant kon inhibitor concentration was observed for
all the enzyme–inhibitor pairs investigated, providing
no evidence for a binding model more complex than
the assumed one. The k
ass
and k
diss
values obtained by
linear regression analysis (Table 1) were used for calcu-
lating the K
i
values. The final K
i
values, which were
corrected for substrate competition, are listed in
Table 1. Cystatin F was observed to be a tight binding
inhibitor of cathepsins F, K, L, V, with K
i
values ran-
ging from 0.17 to 0.35 nm. Surprisingly cathepsin S,
despite being an endopeptidase, was inhibited by cysta-
tin F substantially more weakly, with K
i
¼33 nm,
comparable to the inhibition of the aminopeptidase
cathepsin H (K
i
¼30 nm). In comparison with other
cystatins, cystatin F is a rather slow binding inhibitor
of the cathepsins, characterized by k
ass
values in the
range of 10
6
)10
7
m
)1
, and high k
diss
values in the
range of 10
)3
to 10
)4
for the tightly inhibited cathep-
sins F, K and V. The reason for the considerably
Table 1. Interaction of cystatin F with cysteine proteases. The experimental conditions and methods are described in the Experimental pro-
cedures section. SD, standard deviation. Data from literature are shown for comparison.
Enzyme
K
i
±SD
[nM]
10
4
·k
diss
[s
)1
]
10
)6
·k
ass
[M
)1
Æs
)1
] Substrate
Cathepsin F 0.17 ± 0.05 20 ± 4 12 ± 1 Z-FR-AMC
Cathepsin K 0.35 ± 0.15 11 ± 3 3.2 ± 0.6 Z-FR-AMC
Cathepsin V 0.30 ± 0.15 4.8 ± 1.4 1.6 ± 0.3 Z-FR-AMC
Cathepsin S 33 ± 13 3.7 ± 0.7 0.011 ± 0.002 Z-FR-AMC
Cathepsin H 36 ± 15 0.57 ± 0.2 0.0016 ± 0.00024 H-R-AMC
Cathepsin C > 100 H-SY-bNA
Cathepsin X > 100 Dnp-GFFW
Papain 1.4 ± 0.4 3.5 ± 0.6 0.25 ± 0.03 Z-FR-AMC
Cathepsin L
a
0.31 Z-FR-AMC
Legumain
b
10 Z-AAN-AMC
Cathepsin B
a
>1000 Z-FR-AMC
Papain
a
1.1 Z-FR-AMC
a
[13].
b
[15].
T. Langerholc et al. Cystatin F in U937 cells
FEBS Journal 272 (2005) 1535–1545 ª2005 FEBS 1537

lower inhibition constants for cathepsins S and H was
due mainly to the low k
ass
values.
Colocalization of cystatin F and potential target
enzymes in U937 cells
Using confocal immunofluorescence microscopy, vesi-
cular staining of cystatin F was observed. Colocalization
of cystatin F with the lysosomal proteins LAMP-2
(Fig. 2A) and CD68 (Fig. 2B) revealed at least partial
endosomal ⁄lysosomal localization of cystatin F.
Cathepsins are considered as typical endosomal ⁄
lysosomal enzymes, characterized by a slightly acidic
pH optimum (reviewed in [4]). Cathepsins B, C, H, K,
L, S and X were all found to be expressed in U937
cells. Their amounts varied considerably, as judged by
a semiquantitative approach based on the level of the
fluorescence signal observed and the concentration of
primary antibodies used. However, when subcellular
localization of cystatin F was compared with that of
the cathepsins, cystatin F was found to be colocalized
with cathepsins X and H (Fig. 2C,D), but not with
cathepsins L (Fig. 3A), B, C and K (not shown). The
results for cathepsin S were less clear and showed
partial colocalization of the two proteins (Fig. 3B). As
both primary antibodies for cathepsin F and cystatin
F were of rabbit origin, a different approach was used.
In this approach cathepsin F was tested for possible
colocalization with cathepsin H, but no colocalization
between the two proteases was observed (not shown).
The fact that cathepsin H colocalized with cystatin F,
as described above, suggested that cathepsin F was not
colocalized with cystatin F. Cystatin F was not colo-
calized with cystatin C (Fig. 3C), a typical secreted
type II cystatin.
Discussion
Cystatin F has been known for some years, but its
activity and functional properties have not been com-
pletely determined. However, initial studies revealed an
inhibitory profile that was not typical of other type II
cystatins [13]. Cystatin F, isolated from a baculovirus
expression system, can form disulfide-bonded dimers,
as shown for the inhibitor expressed in Escherichia coli
[12]. This type of dimerization mechanism is different
from general domain-swapping in the cystatin family
[20]. Although both additional cysteines in cystatin F
at positions 1 and 37 (cystatin C numbering) can form
a disulfide bond [13], the cysteine at position 1 has
been suggested to be involved in dimerization of cysta-
tin F [12], similar to cysteine 3 in stefin B [21]. Higher
dithiotreitol concentrations than previously reported
[12] were needed to restore monomers and inhibi-
tory activity under nondenaturing conditions. Loss of
inhibitory potential of dimerized cystatin F can be
explained by blocking of the N-terminal part, disabling
protease access, or by a conformational change result-
ing from disruption of an intramolecular disulfide
bond between cysteines 1 and 37. As dimers have been
observed in U937 cells under physiological conditions
[22], dimerization of cystatin F could be a process
regulating its inhibitory properties.
Screening of proteases for their inhibition showed
that cystatin F is different from other cystatins, both
in terms of specificity and strength of binding to the
target enzyme. Cystatins are generally rather non-
selective inhibitors. An interesting feature of cystatin
F is the 100-fold stronger inhibition of cathepsins F,
L, and V than of cathepsin S. Cathepsin S is closely
related to other endopeptidases of the papain-like
enzyme family, and its crystal structure contains no
pronounced features which would discriminate it from
the related enzymes [23]. Most of our knowledge
about type II cystatins is based on mutagenesis stud-
ies of human cystatin C, where inhibition of cathep-
sin S depends strongly on the Gln55–Gly59 segment
in the first hairpin loop of cystatin C [24]. In con-
trast, substitutions in this wedge-shaped region have
been shown to be of little importance for the inhibi-
tion of cathepsin L [25]. The region Gln55–Gly59 in
cystatin F is the same as in cystatin C, except for an
unfavorable substitution of the nonpolar Ala58 by
Lys. Molecular modeling on the stefin B–papain com-
plex indeed shows steric clashes between Lys58 of
cystatin F and the bulky Tyr18 of cathepsin S. In
contrast, Tyr18 is replaced by the smaller Asn or
Asp in all other known lysosomal cysteine proteases,
indicating easier accommodation of bulky side chains
like that of Lys. This structural feature may in part
contribute to weaker inhibition of cathepsin S by
cystatin F.
The side chain of Val10 in cystatin C, which enters
the S
2
pocket of the cysteine protease, is generally
important for making a strong contribution to the
affinity for cathepsins B, H, L and S [24] and is
replaced by an unfavorable proline in cystatin F, thus
partially explaining the overall lowered affinity of cyst-
atin F for these enzymes. Proline in the S
2
site is a fea-
ture of human stefin A and cystatins F, S and SN, all
of which are significantly less potent inhibitors of cath-
epsin B than cystatin C [9,26]. Unlike Val10, Leu9
which occupies the S
3
pocket in cystatin C is the most
discriminating residue for binding to cathepsins B, H,
L, S [24]. No L9K mutants of cystatin C have been
prepared yet to study the effect of incorporating the
Cystatin F in U937 cells T. Langerholc et al.
1538 FEBS Journal 272 (2005) 1535–1545 ª2005 FEBS
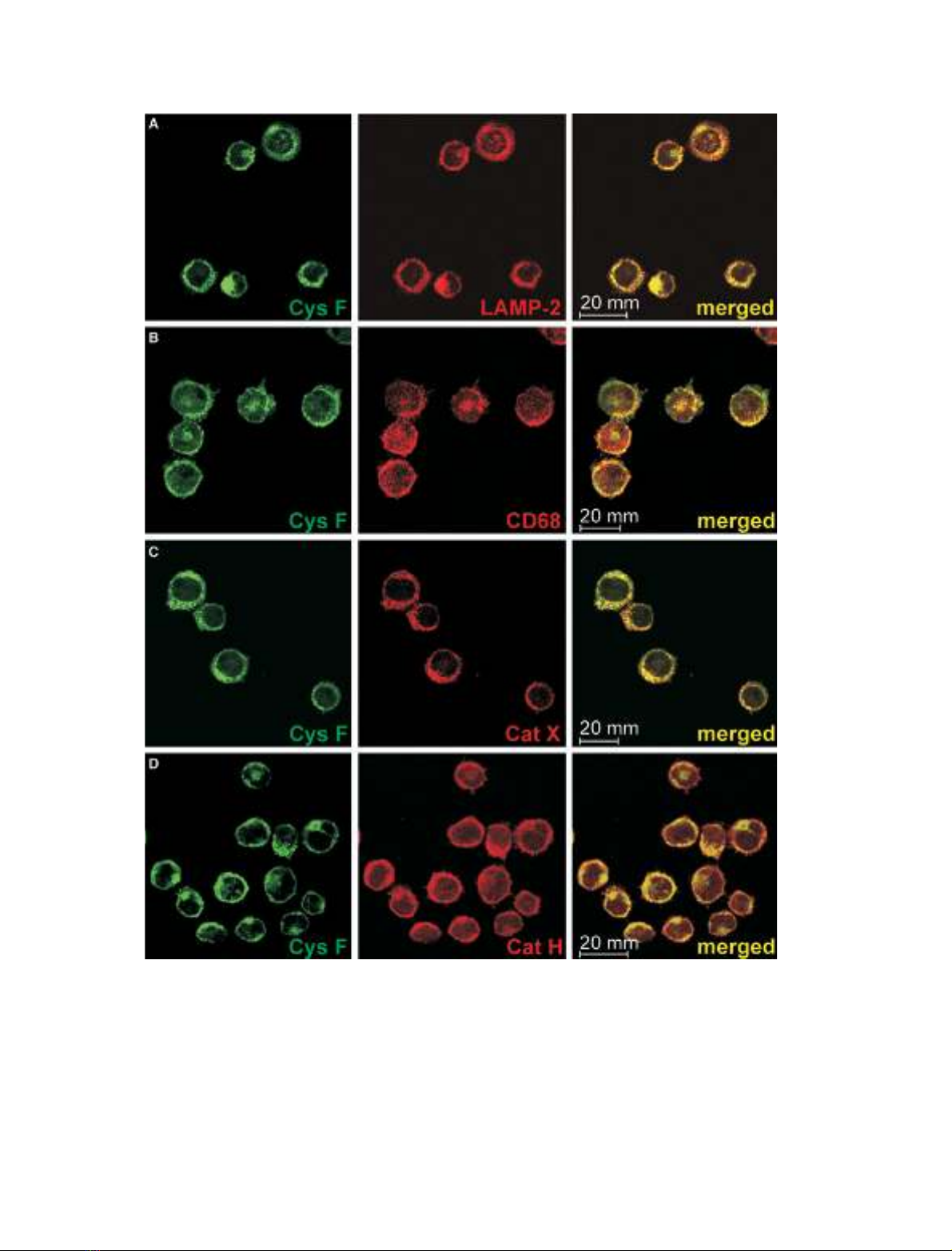
Fig. 2. Immunolabeling of cystatin F in U937 cells, where colocalization was found. Specific monoclonal (mAb) and polyclonal (pAb) antibod-
ies were applied. In all pictures, cystatin F was labeled with primary rabbit anti-(cystatin F) pAb and goat anti-rabbit Alexa Fluor488-labeled
secondary antibody (Ab) (green). Red color originates from labeling with: (A) mouse anti-(LAMP-2) mAb and goat anti-mouse Alexa Fluor
546-labeled secondary Ab; (B) mouse anti-CD68 mAb and goat anti-mouse Alexa Fluor546-labeled secondary Ab; (C) mouse anti-(cathepsin
X) 1F12 mAb and goat anti-mouse Alexa Fluor546-labeled secondary Ab; (D) sheep anti-(cathepsin H) pAb and donkey anti-sheep Alexa
Fluor546-labeled secondary Ab. Before merging the images, signals for red and green fluorescence were adjusted to comparable levels.
The sites of colocalization are shown in yellow.
T. Langerholc et al. Cystatin F in U937 cells
FEBS Journal 272 (2005) 1535–1545 ª2005 FEBS 1539









![Vaccine và ứng dụng: Bài tiểu luận [chuẩn SEO]](https://cdn.tailieu.vn/images/document/thumbnail/2016/20160519/3008140018/135x160/652005293.jpg)












![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)


