Galactosyl-mimodye ligands for
Pseudomonas fluorescens
b-galactose dehydrogenase
Design, synthesis and evaluation
C. F. Mazitsos
1
, D. J. Rigden
2
, P. G. Tsoungas
3
and Y. D. Clonis
1
1
Laboratory of Enzyme Technology, Department of Agricultural Biotechnology, Agricultural University of Athens, Greece;
2
Embrapa Recursos Gene
´ticos e Biotecnologia, Brası´lia, Brazil;
3
Department of Pharmaceutical and Biological Chemistry,
School of Pharmacy, University of London, UK
Protein molecular modelling and ligand docking were
employed for the design of anthraquinone galactosyl-bio-
mimetic dye ligands (galactosyl-mimodyes) for the target
enzyme galactose dehydrogenase (GaDH). Using appro-
priate modelling methodology, a GaDH model was build
based on a glucose-fructose oxidoreductase (GFO) protein
template. Subsequent computational analysis predicted
chimaeric mimodye-ligands comprising a NAD-pseudomi-
metic moiety (anthraquinone diaminobenzosulfonic acid)
and a galactosyl-mimetic moiety (2-amino-2-deoxygalactose
or shikimic acid) bearing an aliphatic linkermolecule. In
addition, the designed mimodye ligands had an appropriate
in length and chemical nature spacermolecule via which
they can be attached onto a chromatographic support
without steric clashes upon interaction with GaDH. Fol-
lowing their synthesis, purification and analysis, the ligands
were immobilized to agarose. The respective affinity adsor-
bents, compared to other conventional adsorbents, were
shown to be superior affinity chromatography materials for
the target enzyme, Pseudomonas fluorescens b-galactose
dehydrogenase. In addition, these mimodye affinity adsor-
bents displayed good selectivity, binding low amounts of
enzymes other than GaDH. Further immobilized dye-lig-
ands, comprising different linker and/or spacer molecules, or
not having a biomimetic moiety, had inferior chromato-
graphic behavior. Therefore, these new mimodyes suggested
by computational analysis, are candidates for application in
affinity labeling and structural studies as well as for purifi-
cation of galactose dehydrogenase.
Keywords: affinity chromatography; biomimetic ligands;
galactose dehydrogenase; molecular modelling; triazine dyes.
Galactose dehydrogenase (GaDH;
D
-galactose: NAD
+
1-oxidoreductase; EC 1.1.1.48) catalyses the dehydrogena-
tion of b-
D
-galactopyranose in the presence of NAD
+
to
D
-galacto-1,5-lactone and NADH, acting on the C1 posi-
tion of the sugar substrate. The enzyme generally shows no
absolute specificity either for NAD
+
,asNADP
+
is also
used, albeit to a lesser degree. Nor is the enzyme specific for
D
-galactose, as
D
-fucose is a better substrate, although other
sugars (e.g.
L
-arabinose, 2-deoxy-
D
-galactose) are less
reactive. The kinetic mechanism is ordered Bi-Bi, with the
NAD
+
binding first to the enzyme [1]. GaDH from
Pseudomonas fluorescens is the best studied example, as it
has been cloned and expressed in Escherichia coli [2] and its
full nucleotide sequence determined [3]. The active macro-
molecule possesses two binding sites [4] and consists of two
identical subunits each of 33 kDa (304 amino-acid residues)
[3]. GaDH from Pseudomonas saccharophila has been
studied to a lesser extent [5], whereas the enzyme has been
identified in plants (e.g. green peas, oranges and Arabidopsis
thaliana), algae (e.g. Iridophycus flaccidum) and several
mammals including humans. No information is available
regarding the catalytic mechanism of GaDH, and its
structure has not been determined experimentally or
modelled.
GaDH is an important analytical tool as at alkaline pH
the product galactonolactone is hydrolysed, so that the
reaction becomes irreversible. The enzyme is therefore
useful for the determination of b-
D
-galactose and
a-
D
-galactose, after the latter is converted to the former
by the application of exogenous mutarotase. GaDH is
also exploited for the determination of lactose; the milk
sugar is hydrolysed by lactase, coupled to GaDH which
acts on the resulting b-
D
-galactose. Despite the utility of
GaDH, a simple and rapid purification method is not
available.
The ability to combine knowledge of X-ray crystallo-
graphic studies, NMR and homology structures with
defined or combinatorial chemical synthesis and advanced
computational tools has made rational design of affinity
ligands more feasible, powerful, logical and faster [6]. In the
present work, rigorous protein molecular modelling was
Correspondence to Y. D. Clonis, Laboratory of Enzyme Technology,
Department of Agricultural Biotechnology, Agricultural
University of Athens, 75 Iera Odos Street, GR-11855 Athens,
Greece. Fax: + 30 210 5294307, Tel.: + 30 210 5294311,
E-mail: clonis@aua.gr
Abbreviations: ADH, alcohol dehydrogenase; BM, biomimetic ligand
or mimodye ligand; CB3GA, Cibacron blue 3GA; GaDH, galactose
dehydrogenase; GaO, galactose oxidase; GFO, glucose-fructose
oxidoreductase; GlDH, glucose dehydrogenase; GlO, glucose oxidase;
VBAR, Vilmafix Blue A-R; CDI, 1,1¢-carbonyldiimidazole.
Enzymes: galactose dehydrogenase (GaDH;
D
-galactose: NAD
+
1-oxidoreductase; EC 1.1.1.48).
(Received 31 May 2002, revised 16 August 2002,
accepted 28 August 2002)
Eur. J. Biochem. 269, 5391–5405 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03211.x

used to create an objectively sound model of GaDH using as
the best available template glucose-fructose oxidoreductase
(GFO). This model was then exploited in the design of novel
galactosyl-biomimetic chlorotriazine dye-ligands (mimodye
ligands) with bifunctional or chimaeric characteristics. In
particular, these galactosyl-mimodye ligands are designed to
bear a structural portion that interacts with the NAD
+
-
binding site and a biomimetic moiety that interacts with the
sugar-binding site of GaDH. The effectiveness of the
bifunctional (chimaeric) ligand concept has been previously
demonstrated with ketocarboxyl- [7,9] and glutathionyl-
biomimetic [10] ligands but never with sugar ones. These
mimodye ligands are expected to become useful tools for the
identification of amino-acid residues of the binding sites of
GaDH after affinity labelling. For this purpose, the
galactosyl-mimodyes were designed to bear a reactive
chloro-triazine structural scaffold, present in all reactive
triazinyl-dye ligands including the archetypal CB3GA and
VBAR. Other mimodyes and certain conventional triazine
dyes are known to act as affinity labels due to their
chlorine(s) atom(s) which react with appropriate residues
of the targeted enzyme active site [11–13]. Furthermore,
when the chlorine was substituted with a carefully chosen
spacer molecule, a nonreactive biomimetic ligand was
obtained which could be immobilized on a chromatography
support. We envisage that these immobilized ligands will be
of great use in the purification of GaDH from different
sources.
EXPERIMENTAL PROCEDURES
Materials
b-Galactose dehydrogenase (EC 1.1.1.48, P. fluorescens
gene expressed in E. coli), galactose oxidase crude lyophi-
lized powder (EC 1.1.3.9, from Dactylium dendroides),
glucose oxidase crude lyophilized powder (EC 1.1.3.4,
from Aspergillus niger, crude),
D
(+)-galactosamine
(2-amino-2-deoxy-
D
-galactopyranose; chondrosamine),
D
(+)-galactose (minimum 99%),
D
(+)-glucose, 1,3-diamino-
2-hydroxypropane, bromoacetic acid N-hydroxysuc-
cinimide ester, e-amino-n-caproic acid, ethylene-diamine,
1,5-diaminopentane, 1,6-hexane-diamine, 1,12-diaminodo-
decane, 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide
(EDAC), 1,1¢-carbonyldiimidazole, o-tolidine, o-dianisi-
dine, lipophilic Sephadex LH-20, CM–Sepharose CL-6B
and DEAE–Sepharose CL-6B were obtained from Sigma
(St Louis, MO, USA). All other diaminoalkanes were
obtained from Aldrich (USA), whereas, shikimic acid was
obtained from Fluka (USA). Peroxidase (from horseradish,
grade I), NAD
+
(crystallized lithium salt c. 100%) and
crystalline bovine serum albumin (fraction V) were obtained
from Boehringer Mannheim (Germany). Hexylamine and
nutrient broth (for microbiology) were obtained from
Merck (Germany). The agarose chromatography gel
Sepharose CL-6B was obtained from Pharmacia. F324
P. fluorescens biovar V1 was kindly donated by G. J.
Nychas (Laboratory of Microbiology and Biotechnology
of Foods, Agricultural University of Athens). Baker’s
yeast, green peas and rabbit liver were purchased at the
local market. Glucose dehydrogenase was extracted from
P. fluorescens and baker’s yeast, while alcohol dehydro-
genase was extracted from baker’s yeast and green peas.
Protein modelling
Fold recognition methods [14–17] were employed to deter-
mine the best template to use for construction of a model of
GaDH. Given the low sequence identity between GaDH
and the GFO template used (17%) a rigorous modelling
strategy was used, as previously (e.g [18,19]). In this way the
challenge of modelling based on low sequence identity was
met with a strategy designed to maximize model accuracy.
Although errors will undoubtedly remain, the probability of
producing a useful model is thereby enhanced. The essential
elements of this strategy are the construction and analysis of
multiple models (20 in this case), derived from limited
randomization of initial coordinates and made with the
program
MODELLER
[20], followed by analysis of packing
and solvent exposure characteristics with
PROSA
II [21]. The
resulting profiles showed regions of unusual protein struc-
ture characteristics as peaks attaining positive values. These
regions may result from locally inaccurate target-template
alignment so that variant alignments, altered in these
doubtful regions, were tested through further cycles of
model construction and analysis. When better
PROSA
results
were obtained for the variant alignment it was assumed to
be more correct than the original. Stereochemical analysis
using
PROCHECK
was also employed, particularly when the
optimal target-template alignment had been reached. Pro-
tein models were visualized using O [22]. Structurally similar
proteins to the template were sought in the FSSP database
(http://www.ebi.ac.uk/dali/fssp) [23].
STRIDE
[24] was used
for the definition of secondary structure.
Ligand design and docking
The ideal biomimetic would combine moieties that bind
both to the cofactor NAD and the substrate binding sites.
The initially considered building elementswere two com-
mercially available compounds: (a) anthrquinone-diamino-
benzosulfonyl-dichlorotriazine (Vilmafix blue A-R or
VBAR) containing three of the four ring systems of the
well known dye Cibacron Blue 3GA (CB3GA), both known
binding mimics of NAD(P) [8,9,25] and (b) 2-amino-
2-deoxygalactose, a substrate of GaDH [1]. Both these
molecules have readily modifiable chemical groups to which
could be attached an appropriate linkermolecule in order
to effect their fusion. 1-Amino-1-deoxygalactose, although
commercially available, was not considered as GaDH
attacks at the C1 position of the substrate, so that this
position was thought better preserved in the ligand.
However, in place of galactose shikimic acid was considered
which, although only moderately structurally similar to
GaDH substrates, has a clear advantage over them in terms
of chemical stability. Finally, a spacermolecule of appro-
priate length and chemical nature was designed to chemi-
cally attach the complete ligand, via its triazine group (ring
3), to the chromatographic matrix.
The HIC-UP database of heterocompounds [26] was
used as a source of the Cibacron Blue-derived, b-
D
-galactose
and shikimic acid components. These were rotated and
translated with respect to the protein model using
O
[22]
until optimal steric and chemical complementarity was
reached. The tendency of Cibacron Blue-like ring systems
to bind in NAD(P) binding sites with anthraquinone
mimicking adenine, along with biochemical data regarding
5392 C. F. Mazitsos et al. (Eur. J. Biochem. 269)FEBS 2002
sugar binding to related enzymes provided useful informa-
tion to guide the docking, as described later. Side chain
reorientations to rotameric conformations were allowed
where they significantly enhanced interactions with ligands.
The mimodye ligands (e.g. BM1 and BM2) were mod-
elled through the fusion of their respective enzyme-bound
components and the resulting complexes refined using
CNS
[27]. Topology and parameter files for energy minimization
of the ligand were generated using
XPLO
2
D
[28] and hand-
edited to reflect ideal stereochemical values.
Synthesis and purification of the dye-ligands
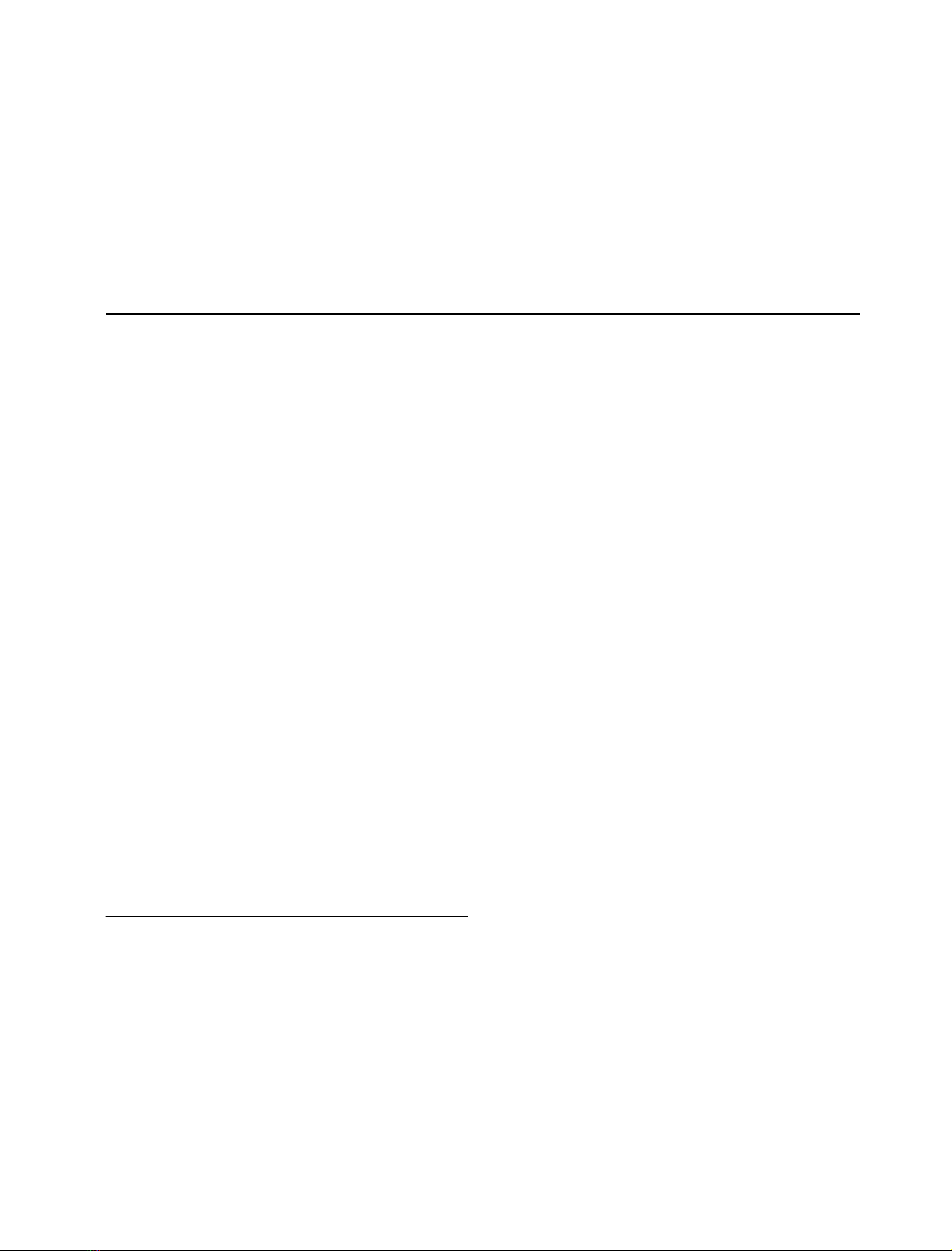
Amino-alkyl-VBAR dyes. (Table 1, structures aVBAR-
fVBAR). Solid commercial VBAR (50 mg, 0.045 mmol
dichloroform, purity 61.3%, w/w) was added to cold water
(2 mL) and the solution was slowly introduced under
stirring to a solution (3 mL) of the alkyl-diamines
(0.73 mmol). The pH was adjusted to 8.9–9.0 and kept at
this value with NaOH (0.1
M
) until the end of the reaction
(2.5–3 h, 25 C). The progress of each reaction was
monitored by TLC (1-butanol-2-propanol-ethylacetate-
wate, 2 : 4 : 1 : 3 v/v/v/v) upon completion of the reaction,
solid NaCl was added (final content 3%, w/v) and the
mixture was left at 4 C. The pH of the mixture was
adjusted with HCl (1
M
) to 1.0 and the precipitate was
filtered (Whatman paper filter 50, hardened), washed with
5mLeachofHCl(1
M
) and cold acetone, then with 7 mL
of diethyl ether and dried under reduced pressure. The solid
dye (approximately 30 mg) was dissolved in 50 : 50 water/
methanol (50%) and dimethylsulfoxide (50%) mixture, and
purified on a lipophilic Sephadex LH-20 column
(30 ·2.5 cm) [29]. The purified product was stored in a
desicator at 4 C.
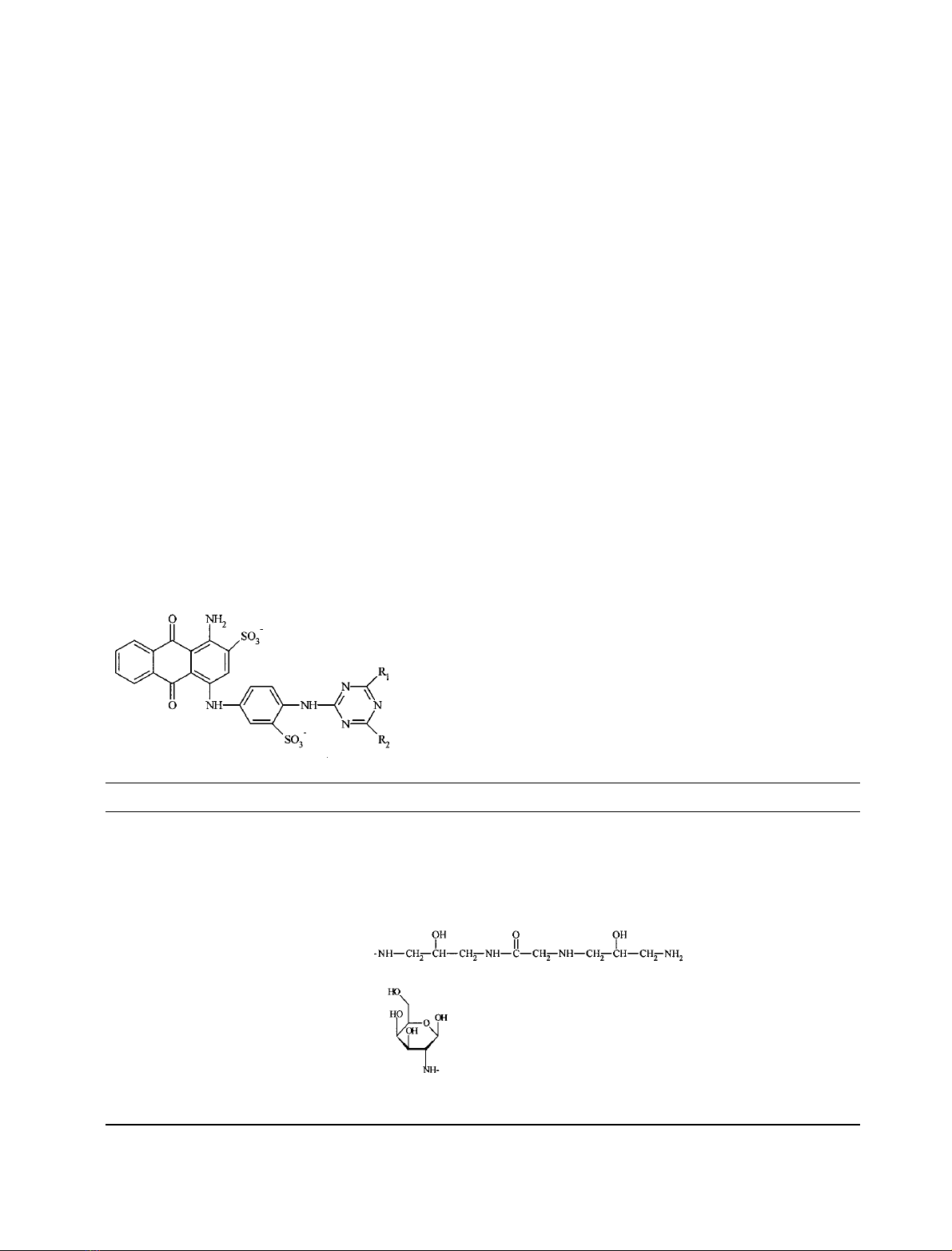
Hydrophilic spacer-VBAR dye. (Table 1, structure
gVBAR; Fig. 1). Stage 1: solid commercial VBAR
(20 mg, 0.018 mmol dichloroform, purity 61.3%, w/w)
was added to cold water (1 mL) and the solution was slowly
introduced under stirring to 1,3-diamino-2-hydroxypropane
(3 mL, 0.29 mmol). The pH was adjusted to 8.9–9.0 and
kept at this value with NaOH (0.1
M
) until the end of the
reaction (2.5–3 h, 25 C). The progress of the reaction was
monitored by TLC (1-butanol-2-propanol-ethylacetate-
water, 2 : 4 : 1 : 3 v/v/v/v). Upon completion of the
reaction, the dye was purified according to the method
already described (see above). Stage 2: the purified product,
1,3-diamino-2-hydroxypropano-VBAR, was dissolved in
dimethylsulfoxide/water (3 mL, 50 : 50, v/v) and the pH
was adjusted to 7.5 with NaOH (0.1
M
). 0.2 mmol of
bromoacetic acid N-hydroxysuccinimide ester [30,31]
were dissolved in dioxane (1 mL) and this solution was
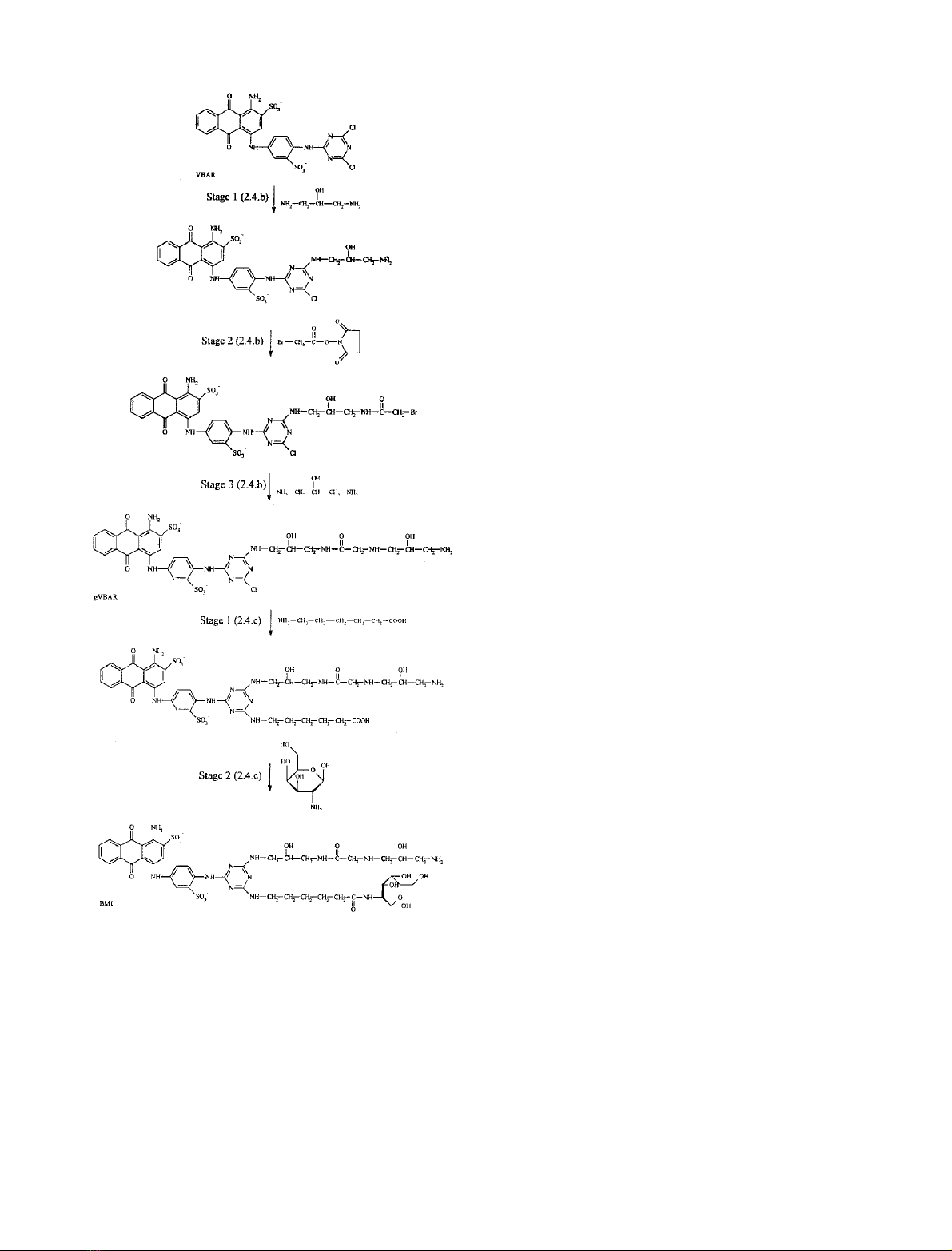
Table 1. The structures of amino-alkyl-VBAR dyes (a-fVBAR), hydrophilic spacer-VBAR dye (gVBAR), galactosamine-VBAR dye and archetypal
VBAR dye.
Ligand R
1
R
2a
aVBAR –NH-(CH
2
)
2
-NH
2
–NH
2
bVBAR –NH-(CH
2
)
4
-NH
2
–NH
2
cVBAR –NH-(CH
2
)
6
-NH
2
–NH
2
dVBAR –NH-(CH
2
)
8
-NH
2
–NH
2
eVBAR –NH-(CH
2
)
10
-NH
2
–NH
2
fVBAR –NH-(CH
2
)
12
-NH
2
–NH
2
gVBAR –NH
2
Galactosamine-VBAR
b
–Cl
VBAR –Cl (– NH
2
)
a
–Cl
a
Following ligand immobilization, the -NH
2
group has replaced the -Cl atom.
b
The galactosamine-VBAR dye was synthesized employing
the procedure for amino-alkyl-VBAR dyes but using the amino-sugar instead the diamino-alkane.
FEBS 2002 Galactosyl-mimodyes for galactose dehydrogenase (Eur. J. Biochem. 269) 5393
introduced to the dye solution. The pH was maintained to
7.5 until the end of the reaction (1.5 h, 4 C, as judged by
TLC). The progress of the reaction was monitored by TLC
(1-butanol-2-propanol-ethylacetate-water, 2 : 4 : 1 : 3 v/v/
v/v). Upon completion of the reaction, the mixture was
lyophilized and the dye was purified on the lipophilic
Sephadex LH-20 column [29]. Stage 3: the purified product,
bromoacetylated 1,3-diamino-2-hydroxypropano-VBAR,
was dissolved in 0.1
M
NaHCO
3
, pH 9.0 (2 mL) and the
solution was slowly introduced under stirring to a solution
of 0.4
M
1,3-diamino-2-hydroxypropane in 0.1
M
NaHCO
3
,
pH 9.0 (2 mL), while maintaining the pH to 9.0 with HCl
(1
M
). The solution was then left under stirring for another
48–72 h (25 C), without further adjustment of the pH. The
progress of the reaction was monitored by TLC (1-butanol-
2-propanol-ethylacetate-water, 2 : 4 : 1 : 3 v/v/v/v). Upon
completion of the reaction, the dye was purified according
to the method already described (see above).
Biomimetic dye BM1. (Table 2, structure BM1; Fig. 1)
Stage 1: purified hydrophilic spacer-VBAR, structure g
(approx. 15 mg, 0.017 mmol) was dissolved in dimethyl-
sulfoxide/water (3 mL, 50 : 50, v/v) and the solution was
introduced under stirring to e-amino-n-caproic acid (2 mL,
0.17 mmol). The pH was adjusted to 9.0 and the mixture
was left shaking at 60 C for 3 h. The progress of the reaction
was monitored by TLC (1-butanol-2-propanol-ethylacetate-
water, 2 : 4 : 1 : 3 v/v/v/v). Upon completion of the
reaction, the dye was purified according to the method
already described (see above). Control dye C
6
gVBAR
(Table 2) was synthesized in the same way. Stage 2: the
purified product obtained from stage 1, was dissolved in
dimethylsulfoxide/water (3 mL, 50 : 50, v/v), introduced to
a solution of
D
(+)-galactosamine (3 mL, 0.62 mmol), and
the pH was adjusted to 4.6, before freshly prepared solution
of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (0.3 mL,
250 mg) was introduced dropwise under stirring over a
period of 5 min, while maintaining the pH at 4.6–5.0. The
reaction was stirred for 20 h at 25 C without pH
adjustment and monitored by TLC (1-butanol-2-propanol-
ethylacetate-water, 2 : 4 : 1 : 3 v/v/v/v). A silver nitrate
ammonia solution was used as a spray reagent for detecting
the galactose-analogue in the newly synthesized dye [32].
The product, structure BM1, was precipitated by addition
of solid NaCl (final content 15%, w/v), filtered and washed
with 7 mL of NaCl solution (15%, w/v) and 5 mL of cold
acetone, and dried under reduced pressure. The product was
re-suspended in 2 mL of water and precipitated by addition
of solid NaCl (final content 10%, w/v). The precipitate was
filtered and washed with 7 mL each of NaCl solution (10%,
w/v) and cold acetone, desiccated with 7 mL of diethyl ether
and dried under reduced pressure.
Biomimetic dye BM2. (Table 2, structure BM2; Fig. 2).
Stage 1: solid commercial VBAR (20 mg, 0.018 mmol
dichloroform, purity 61.3%, w/w) was added to cold water
(1 mL) and the solution was slowly introduced under
stirring to a solution (3 mL) of 1,3-diaminopropane
(0.29 mmol). The pH was adjusted to 8.9–9.0 and kept at
this value with NaOH (0.1
M
) until the end of the reaction
(2.5–3 h, 25 C). The progress of each reaction was
monitored by TLC (1-butanol-2-propanol-ethylacetate-
water, 2 : 4 : 1 : 3 v/v/v/v). Upon completion of the
reaction, the dye was purified according to the method
already described (see above). Stage 2: the purified product,
VBAR-1,3-diaminopropane, was dissolved in dimethylsulf-
oxide/water (3 mL, 50 : 50, v/v), introduced to a solution of
shikimic acid (3 mL, 0.62 mmol), and the pH was adjusted
to 4.6, before freshly prepared solution of 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide (0.3 mL, 250 mg) was
introduced dropwise under stirring over a period of 5 min,
while maintaining the pH at 4.6–5.0. The reaction was
stirred for a further 20 h at 25 C without pH adjustment
and monitored by TLC (1-butanol-2-propanol-ethylacetate-
Fig. 1. Steps for the synthesis of gVBAR dye and of mimodye BM1.
5394 C. F. Mazitsos et al. (Eur. J. Biochem. 269)FEBS 2002
water, 2 : 4 : 1 : 3 v/v/v/v). The product, VBAR-1,3-diami-
nopropano-shikimic acid, was precipitated by addition of
solid NaCl (final content 15%, w/v), filtered and washed
with 7 mL of NaCl solution (15%, w/v) and 5 mL of cold
acetone, and dried under reduced pressure. The product was
dissolved in a 50 : 50 water:methanol (50%) and dimeth-
ylsulfoxide (50%) mixture, and purified to homogeneity on
a lipophilic Sephadex LH-20 column (30 ·2.5 cm) [29].
Control dyes C
6
NgVBAR and C
3
NgVBAR (Table 2) were
synthesizedinthesamewasasinstages1and2.Stages3–5:
the purified product, VBAR-1,3-diaminopropano-shikimic
acid, was dissolved in dimethylsulfoxide/water (3 mL,
50 : 50, v/v/v) and the solution was introduced under
stirring to 1,3-diamino-2-hydroxypropane (2 mL,
0.17 mmol). The pH was adjusted to 9.0 and the mixture
was left shaking at 60 C for 3 h. The progress of the reaction
was monitored by TLC (1-butanol-2-propanol-ethylacetate-
water, 2 : 4 : 1 : 3 v/v/v/v). Upon completion of the
reaction, the dye was purified according to the method
already described (as above). The purified product, 1,3-
diamino-2-hydroxypropano-VBAR-1,3-diaminopropano-
shikimic acid, was dissolved in dimethylsulfoxide/water
(3 mL, 50 : 50, v/v) and the pH was adjusted to 7.5 with
NaOH (0.1
M
). 0.2 mmol of bromoacetic acid N-hydroxy-
succinimide ester were dissolved in dioxane (1 mL) and this
solution was introduced to the dye solution. The pH was
maintained at 7.5 until the end of the reaction (1.5 h, 4 C,
as judged by TLC). The progress of the reaction was
monitored by TLC (1-butanol-2-propanol-ethylacetate-
water, 2 : 4 : 1 : 3 v/v/v/v). Upon completion of the
reaction, the mixture was lyophilized and the dye was
purified by applying preparative TLC as follows: lyophilized
reaction mixture was dissolved in dimethylsulfoxide/water
(0.4 mL, 50 : 50, v/v) and the solution applied on a
Kieselgel 60 plate (silica gel 60, 0.2 mm, 20 ·20 cm,
Merck). The plate was developed using a 1-butanol-2-
propanol-ethylacetate-water (2 : 4 : 1 : 3 v/v/v/v) mixture.
Following completion of the chromatography, the plate was
dried and the band of interest was scraped off. The desired
dye was extracted from the silica gel with water, filtered
through a Millipore cellulose membrane filter (0.45 lm pore
size) and lyophilized. The purified product, bromoacetylated
1,3-diamino-2-hydroxypropano-VBAR-1,3-diaminopropano-
shikimic acid, was dissolved in 2 mL of 0.1
M
NaHCO
3
,
pH 9.0, and the solution was slowly introduced under
stirring to a 2-mL solution of 0.4
M
1,3-diamino-2-hydroxy-
propane in 0.1
M
NaHCO
3
, pH 9.0 (the pH maintained at 9
using 1
M
HCl). The solution was then left under stirring for
another 48–72 h (25 C), without further adjustment of the
pH. The progress of the reaction was monitored by TLC
(1-butanol-2-propanol-ethylacetate-water, 2 : 4 : 1 : 3 v/v/
v/v). Upon completion of the reaction, the product,
hydrophilic spacer-VBAR-1,3-diaminopropano-shikimic
acid, was precipitated by addition of solid NaCl (final
content 15%, w/v), filtered and washed with 7 mL of NaCl
solution (15%, w/v) and 5 mL of cold acetone, and dried
under reduced pressure. The product was re-suspended in
2 mL of water and precipitated by addition of solid NaCl
(final content 10%, w/v). The precipitate was filtered
and washed with 7 mL each of NaCl solution (10%, w/v)
and cold acetone, desiccated with 7 mL of diethyl ether and
dried under reduced pressure.
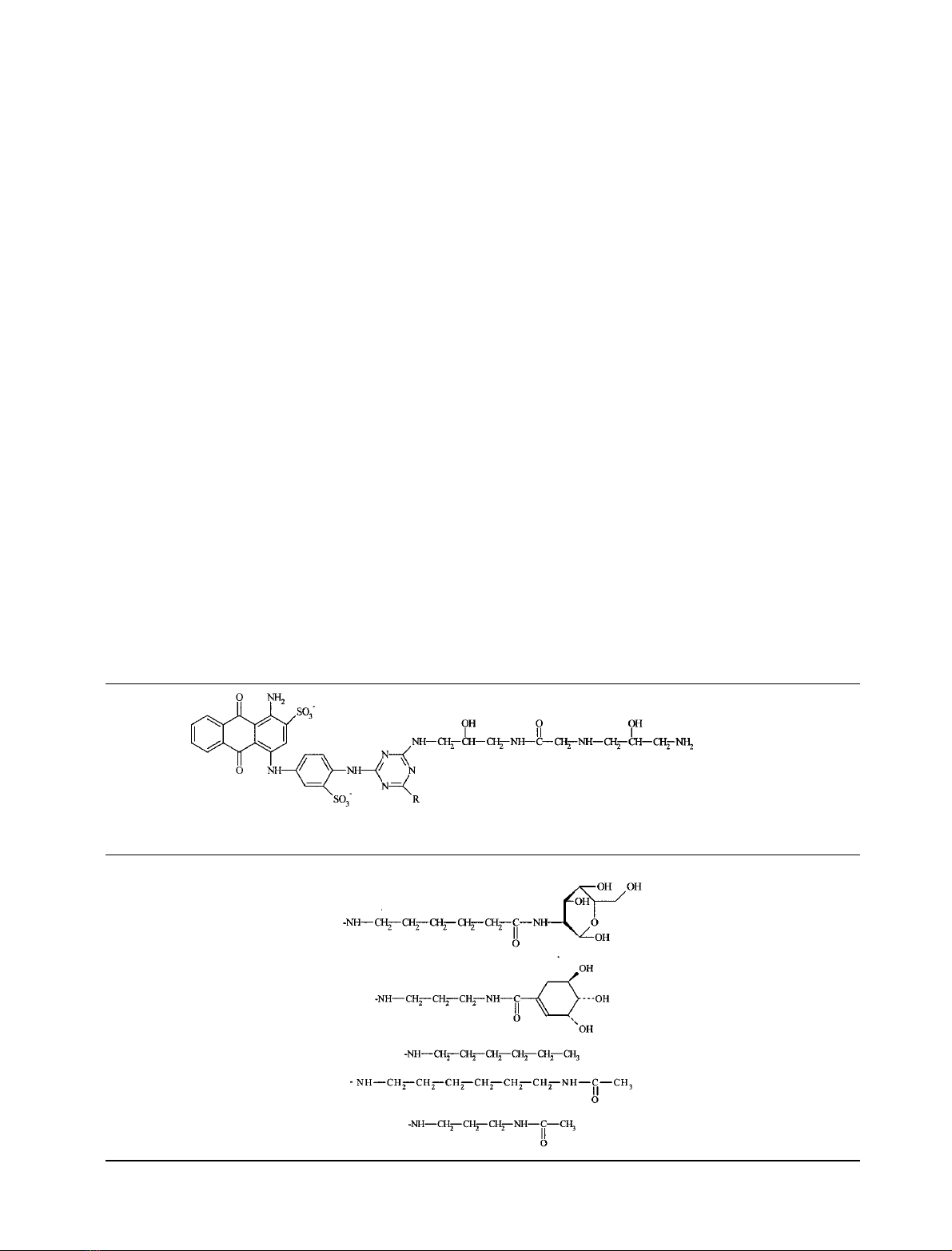
Table 2. The structures of the mimodyes BM1 and BM2 and the control dyes.
Dye-ligand –R
BM1
BM2
C
6
gVBAR
C
6
NgVBAR
C
3
NgVBAR
FEBS 2002 Galactosyl-mimodyes for galactose dehydrogenase (Eur. J. Biochem. 269) 5395






















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)


