SỞ GD & ĐT ĐIỆN BIÊN
TRƯỜNG THCS VÀ THPT QUÀI T
ĐỀ KIỂM TRA GIỮA HỌC KỲ II
MÔN: HÓA HỌC LỚP 10
NĂM HỌC 2022 – 2023
ĐỀ CHÍNH THỨC
(Đề có 4 trang)
Thời gian:45 phút (không kể thời gian phát đề)
Họ và tên: .................................................................Lớp: 10A…
Mã đề 102
Điểm Lời phê của thầy, cô giáo
PHẦN TÔ TRẮC NGHIỆM
1 2 3 4 5 6 7 8 9 10 11 12 13 14
ⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶ
ⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷ
ⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸ
ⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹ
15 16 17 18 19 20 21 22 23 24 25 26 27 28
ⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶⒶ
ⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷⒷ
ⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸⒸ
ⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹⒹ
I. Phần trắc nghiệm ( 7 điểm)
Câu 1: Phản ứng sau thuộc loại phản ứng nào?
C 2H4(g) + H2 → C2H6(g) =−137,0kJ
A. Phản ứng tỏa nhiệt; B. Phản ứng thu nhiệt;
C. Vừa thu nhiệt, vừa tỏa nhiệt; D. Không thuộc loại nào.
Câu 2: Số oxi hoá là một số đại số đặc trưng cho đại lượng nào sau đây của nguyên tử trong phân
tử?
A. Hoá trị. B. Số hiệu.
C. Khối lượng. D. Điện tích.
Câu 3: Trong phản ứng oxi hóa - khử, chất oxi hóa là chất
A. nhường proton. B. nhận proton.
C. nhường electron. D. nhận electron.
Câu 4: Giá trị tuyệt đối của biến thiên enthalpy càng lớn thì
A. nhiệt lượng tỏa ra hay thu vào của phản ứng càng ít.
B. nhiệt tỏa ra càng nhiều và nhiệt thu vào càng ít.
C. nhiệt tỏa ra càng ít và nhiệt thu vào càng nhiều.
D. nhiệt lượng tỏa ra hay thu vào của phản ứng càng nhiều
Câu 5: Số oxi hóa của N trong phân tử HNO3là
A. +4. B. +5. C. +2. D. –2.
Câu 6: Fe2O3là thành phần chính của quặng hematite đỏ, dùng để luyện gang. Số oxi hoá của iron
(sắt) trong Fe2O3là:
Trang 1/5 - Mã đề 102
A. -3. B. 3. C. +3. D. 3+.
Câu 7: Hiện tượng thực tiễn nào sau đâykhôngphải phản ứng oxi hóa - khử?
A. Sắt bị han gỉ; B. Mưa.
C. Sản xuất acid sunfuric; D. Đốt cháy than trong không khí;
Câu 8: Phản ứng nào sau đây là phản ứng oxi hóa – khử?
A. CaO + CO2→CaCO3.B. CuO +2HCl→CuCl2+ H2O.
C. CO2+ 2NaOH→Na2CO3+ H2O. D. Fe2O3+ 3CO 2Fe+ 3CO2.
Câu 9: Phương trình nhiệt hóa học giữa nitrogen và oxygen như sau:
N2(g) + O2(g) 2NO(g) = +180 kJ
Kết luận nào sau đây đúng?
A. Phản ứng tỏa nhiệt.
B. Nitrogen và oxygen phản ứng mạnh hơn khi ở nhiệt độ thấp.
C. Phản ứng xảy ra thuận lợi ở điều kiện thường.
D. Phản ứng hóa học xảy ra có sự hấp thụ nhiệt năng từ môi trường.
Câu 10: Pha viên sủi vitamin C vào nước, khi viên sủi tan, thấy nước trong cốc mát hơn, đó là do
A. xảy ra phản ứng oxi hóa – khử. B. xảy ra phản ứng thu nhiệt.
C. xảy ra phản ứng tỏa nhiệt. D. xảy ra phản ứng trung hòa.
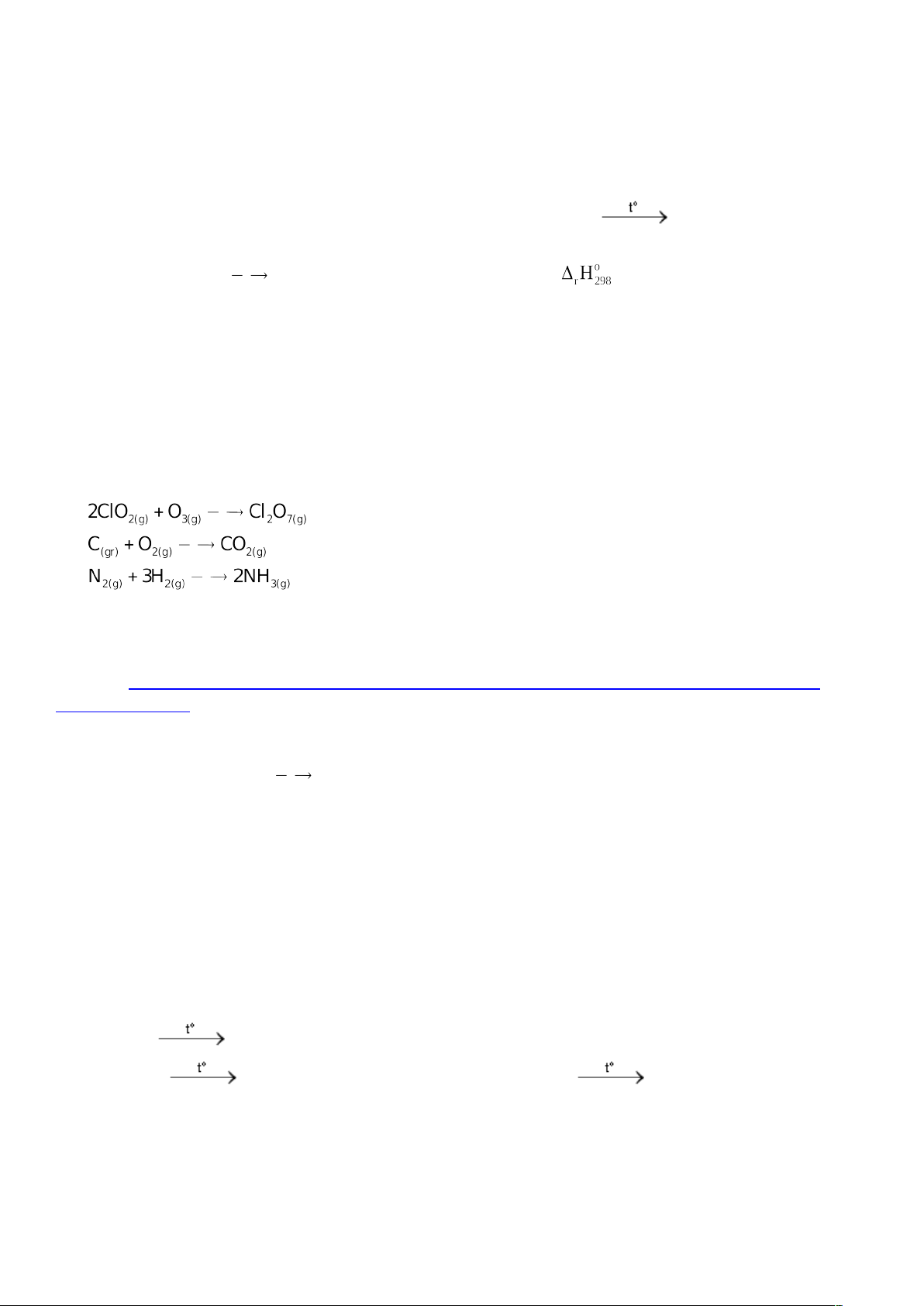
Câu 11: Cho các phương trình nhiệt hóa học sau đây:
(1) ; ∆H1 = -75,7 kJ/mol
(2) ; ∆H2 = -393,5 kJ/mol;
(3) ; ∆H3 = -46,2 kJ/mol
(4)
2(k) (k)
O 2O
; ∆H4 = 498,3 kJ/mol
Số quá trình tỏa nhiệt là
A. 3. B. 4. C. 2. D. 1.
Câu 12: Phản ứng xảy ra khi pin được sử dụng trong điện thoại, máy tính, … giải phóng năng
lượng dưới dạng
A. nhiệt năng. B. hóa năng. C. điện năng. D. cơ năng.
Câu 13: Phản ứng của 1 mol ethanol lỏng với oxygen xảy ra theo phương trình:
C2H5OH(l) + O2(g) CO2(g) + H2O(l)
Cho các phát biểu sau:
(a) Đây là phản ứng tỏa nhiệt vì nó tạo ra khí CO2 và nước lỏng.
(b) Đây là phản ứng oxi hóa – khử với tổng hệ số cân bằng trong phương trình hóa học là 9.
(c) Biến thiên enthalpy chuẩn của phản ứng sẽ thay đổi nếu nước được tạo ra ở thể khí.
(d) Sản phẩm của phản ứng chiếm một thể tích lớn hơn so với chất phản ứng.
Số phát biểu đúng là
A. 3. B. 4. C. 1. D. 2.
Câu 14: Phản ứng oxi hóa khử nào xảy ra trong câu ca dao sau:
“Lúa chiêm lấp ló đầu bờ
Hễ nghe tiếng sấm phất cờ mà lên”
A. C+ O2 CO2.B. CaCO3 + CO2+ H2O→Ca(HCO3)2.
C. N2+ O2 2NOD. 4Fe + 3O2 2Fe2O3.
Câu 15: Cho các phát biểu sau:
(a) Biến thiên enthalpy chuẩn của một phản ứng hóa học là lượng nhiệt kèm theo phản ứng đó ở áp
suất 1 atm và 25 0C.
(b) Nhiệt (tỏa ra hay thu vào) kèm theo một phản ứng được thực hiện ở 1 bar và 298 K là biến thiên
enthalpy chuẩn của phản ứng đó.
(c) Một số phản ứng khi xảy ra làm môi trường xung quanh lạnh đi là do các phản ứng này thu nhiệt
Trang 2/5 - Mã đề 102

và lấy nhiệt từ môi trường.
(d) Một số phản ứng khi xảy ra làm môi trường xung quanh nóng lên là phản ứng thu nhiệt.
Số phát biểu không đúng là
A. 4. B. 1. C. 2. D. 3.
Câu 16: Số oxi hóa của đơn chất luôn bằng
A. +1 B. -2 C. 0D. -1.
Câu 17: Cho phản ứng:
2ZnS (s) + 3O2(g) 2CO2(g) + 4H2O (l) = -285,66 kJ
Xác định giá trị của khi lấy gấp 3 lần khối lượng của các chất phản ứng.
A. –285,66 kJ. B. –1142,64 kJ. C. –571,32 kJ. D. –856,98 kJ.
Câu 18: Hàm lượng iron (II) sulfate trong mẫu nước được xác định qua phản ứng oxi hóa – khử với
potassium permanganate:
FeSO4 + KMnO4 + H2SO4 → Fe2(SO4)3 + MnSO4 + K2SO4 + H2O
Sau khi cân bằng (với hệ số là các số nguyên, tối giản), tổng hệ số của các chất tham gia phản ứng là
A. 22. B. 20. C. 24. D. 28.
Câu 19: Điều kiện nào sau đây không phải là điều kiện chuẩn?
A. Áp suất 1 bar và nhiệt độ 25 0C.
B. Áp suất 1 bar và nhiệt độ 25K.
C. Áp suất 1 bar và nhiệt độ 298 K.
D. Áp suất 1 bar và nhiệt độ 25 0C hay 298 K.
Câu 20: Nhiệt lượng tỏa ra hay thu vào của một phản ứng ở một điều kiện xác định được gọi là gì?
A. Biến thiên enthalpy; B. Biến thiên năng lượng.
C. Nhiệt lượng tỏa ra; D. Nhiệt lượng thu vào;
Câu 21: Phản ứng oxi hóa – khử là phản ứng có sự nhường và nhận
A. proton. B. neutron. C. cation. D. electron.
Câu 22: Enthalpy tạo thành chuẩn (nhiệt tạo thành chuẩn) có kí hiệu là :
A. . B. . C. . D. .
Câu 23: Trong hợp chất SO3, số oxi hoá của sulfur (lưu huỳnh) là
A. +5. B. +6. C. +2. D. +3.
Câu 24: Phản ứng tỏa nhiệt là
A. phản ứng thu năng lượng dưới dạng nhiệt.
B. phản ứng trong đó có tạo thành chất khí hoặc kết tủa
C. phản ứng tỏa năng lượng dưới dạng nhiệt.
D. phản ứng trong đó có sự trao đổi electron.
Câu 25: Sản xuất gang, khí CO khử Fe2O3ở nhiệt độ cao theo phản ứng:
Fe2O3 + 3CO 2Fe + 3CO2. Trong phản ứng trên, chất oxi hóa là
A. Fe. B. CO2.C. CO. D. Fe2O3.
Câu 26: Phát biểu nào sau đây làsai?
A. Số oxi hóa của Othườnglà -2;
B. Số oxi hóa của kim loại kiềm nhóm IA là -1;
C. Số oxi hóa của Hthườnglà +1;
D. Số oxi hóa của kim loại kiềm thổ nhóm IIA là +2.
Câu 27: Phản ứng thu nhiệt có :
A. .B. .C. .D. .
Câu 28: Số oxi hóa của nguyên tử P trong hợp chất P2O5 là
A. 0. B. +2. C. +3. D. +5.
II. Phần tự luận (3,0 điểm)
Câu 1: Cân bằng các phản ứng oxi hóa khử sau theo phương pháp thăng bằng electron:
Trang 3/5 - Mã đề 102

Al + H2SO4 (đặc) Al2(SO4)3 + SO2 + H2O
Câu 2: Xác định biến thiên enthalpy của phản ứng sau ở điều kiện chuẩn:
4FeS(s) + 7O2(g) 2Fe2O3(s) + 4SO2(g)
Hợp chất FeS(s) Fe2O3(s) SO2(g)
∆rH0298 (kJ/mol) –100,0 –825,5 -296,80
------ HẾT ------
BÀI LÀM PHẦN TỰ LUẬN
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
.................................................................................................................................................................
Trang 4/5 - Mã đề 102

Trang 5/5 - Mã đề 102



![Đề thi Tiếng Anh có đáp án [kèm lời giải chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250810/duykpmg/135x160/64731754886819.jpg)




![Đề thi học kì 2 Vật lý lớp 11: Đề minh họa [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250709/linhnhil/135x160/711752026408.jpg)







