Physical properties and dyeability of silk fibers degummed with citric acid
Md. Majibur Rahman Khan
a
, Masuhiro Tsukada
b,*
, Yasuo Gotoh
b
, Hideaki Morikawa
b
, Giuliano Freddi
c
,
Hideki Shiozaki
d
a
Department of Biosystems Engineering, University of Manitoba, Winnipeg, Manitoba, Canada R3T 5V6
b
Faculty of Textile Science and Technology, Shinshu University, Tokida 3-15-1, Ueda, Nagano 386-856, Japan
c
Stazione Sperimentale per la Seta, Via G. Colomob 83, 20133 Milano, Italy
d
Research Institute for Industrial Art of Kanagawa, Odawara City, Kanagawa 250-0055, Japan
article info
Article history:
Received 11 August 2009
Received in revised form 29 April 2010
Accepted 31 May 2010
Available online 2 July 2010
Keywords:
Biopolymer silk
Citric acid
Degumming ratio
Structure and properties
Dyeability
abstract
Silk fibers from Bombyx mori silkworm was degummed with different concentration of citric acid, and the
physical properties and fine structure were investigated to elucidate the effects of citric acid treatment.
The silk sericin removal percentage was almost 100% after degumming with 30% citric acid which
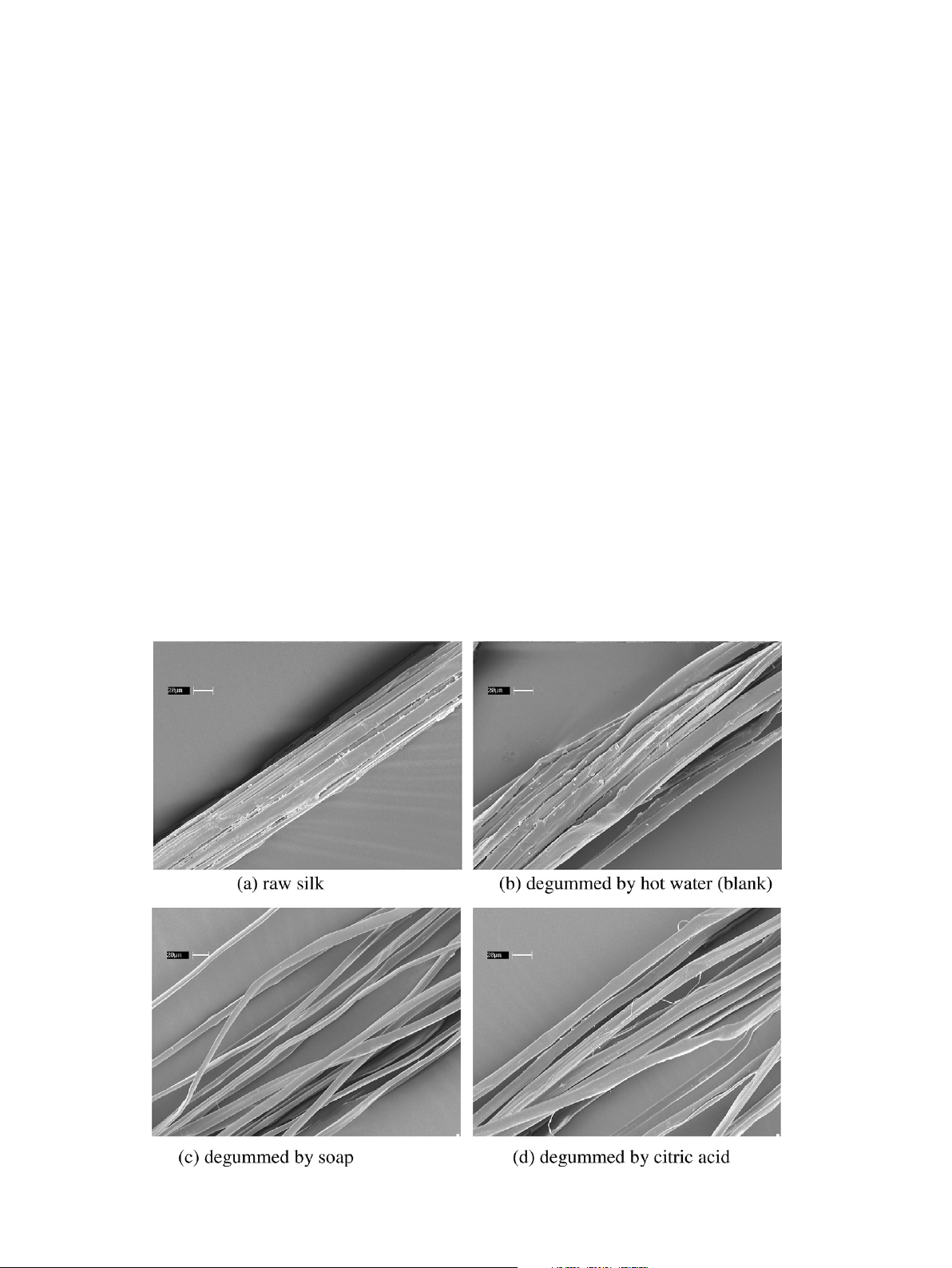
resulted in a total weight loss of 25.4% in the silk fibers. The surface morphology of silk fiber degummed
with citric acid was very smooth and fine, showed perfect degumming like traditional soap-alkali
method. The tensile strength of silk fiber was increased after degumming with citric acid (507 MPa),
where as the traditional soap-alkali method causes to decrease the strength about half of the control silk
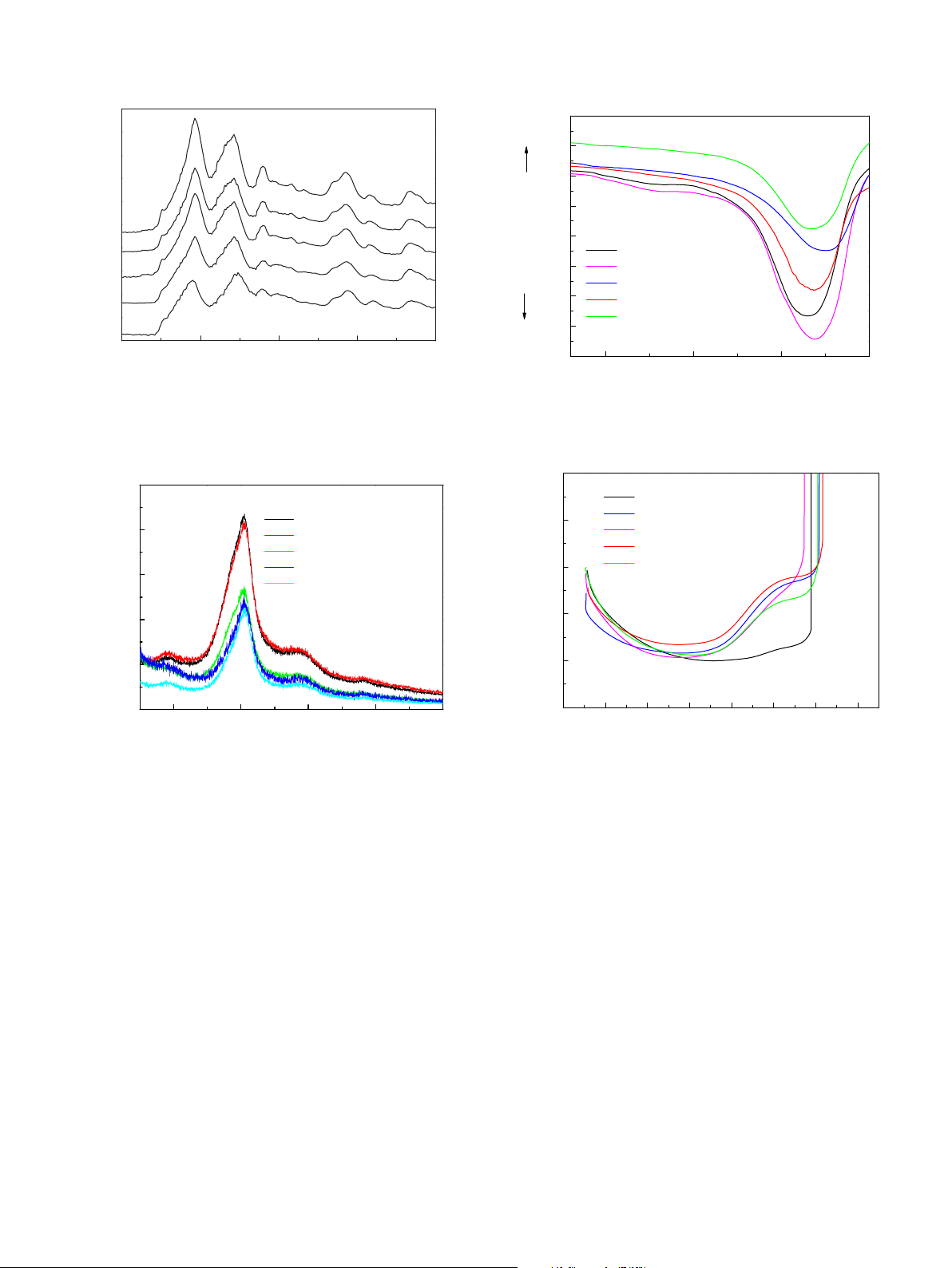
fiber (250 MPa). The molecular conformation estimated by Fourier transform infrared spectroscopy and
the crystalline structure evaluated from X-ray diffraction curve stayed unchanged regardless of the
degumming with citric acid and soap. The dye uptake percentage of silk fiber degummed with citric acid
decreased slightly, about 4.2%. On the other hand, the dye uptake percentage of silk degummed with soap
was higher which indicates the disordering of the molecular orientation of the laterally ordered structure,
accompanied with the partial hydrolysis of silk fibroin molecules by the alkali action of soap. The thermal
properties were greatly enhanced by soap and citric acid degumming agents. Dynamic mechanical ther-
mal analysis showed silk degummed with citric acid is more stable in higher temperature than that of
soap. With heating at above 300 °C, the silk degummed with citric acid shows an increase in storage mod-
ulus and an onset of tan dpeaks at 325 °C and the melt flow of the sample was inhibited. The degumming
of silk fibers with citric acid is safe and the results obtained are quite promising as a basis for possible
future industrial application.
Ó2010 Elsevier Ltd. All rights reserved.
1. Introduction
Silk fiber is one of the most familiar, as well as being a very use-
ful biopolymer and is universally acclaimed for most of the desir-
able properties of textile fiber: fineness, strength, elasticity,
dyeability, softness, flexibility, smooth feeling, luster, elegance,
grace and high rating (Trotman, 1975; Shinohara, 2000). It is the
only commercially available natural fiber in continuous filament
form, produced by the larva of some insects, especially silkworms.
Several species of silk spinning insects exist in nature. However,
silk thread spun by the larvae of silkworm, Bombyx mori (B. mori)
is of practical importance as a source of textile grade fibers.
The silkworm cocoon silk fiber is composed of two cores of
fibroin surrounded by a layer of sericin in a structure known as a
bave (each individual fibroin core is known as a brin) (Pe’rez-
Rigueiro et al., 2000). Fibroin is the structural protein of silk fiber,
whereas sericin is the water soluble proteinaceous glue that serves
to bond the fibers together. The majority of fibroin’s composition is
highly periodic, with simple repeating sections broken by more
complex regions containing amino acids with bulkier side chains.
The highly repetitive sections are composed of glycine (45%), ala-
nine (30%), and serine (12%) in a roughly 3:2:1 ratio and dominated
by [Gly-Ala-Gly-Ala-Gly-Ser]
n
sequences. Fibroin is known to form
mainly three kinds of conformations: silk I with a helical confor-
mation, silk II with an antiparallel b-sheet, and a random coil with-
out definite orders. Sericin contains glycine, serine, and aspartic
acid totaling over 60% of the overall composition (Zhang et al.,
2002; Kaplan et al., 1997; Lotz and Colonna Cesari, 1979).
Silk processing from cocoons to the finished clothing materials
consists of a series of steps which include: reeling, weaving,
degumming, dyeing or printing, and finishing (Zahn, 1993). Silk
sericins play important role in the silk reeling, finishing, and
weaving process. When silk fiber is finally used as textile products,
0960-8524/$ - see front matter Ó2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.biortech.2010.05.100
*Corresponding author. Tel.: +81 268 21 5355; fax: +81 268 21 5388.
E-mail addresses: tsukada@shinshu-u.ac.jp,majib@hotmail.com (M. Tsukada).
Bioresource Technology 101 (2010) 8439–8445
Contents lists available at ScienceDirect
Bioresource Technology
journal homepage: www.elsevier.com/locate/biortech