
MINIREVIEW
Endovanilloids
Putative endogenous ligands of transient receptor potential vanilloid 1 channels
Mario van der Stelt and Vincenzo Di Marzo
Endocannabinoid Research Group, Istituto di Chimica Biomolecolare, Consiglio Nazionale delle Ricerche, Pozzuoli, Italy
Endovanilloids are defined as endogenous ligands of the
transient receptor potential vanilloid type 1 (TRPV1)
protein, a nonselective cation channel that belongs to the
large family of TRP ion channels, and is activated by
the pungent ingredient of hot chilli peppers, capsaicin.
TRPV1 is expressed in some nociceptor efferent neurons,
where it acts as a molecular sensor of noxious heat and
low pH. However, the presence of these channels in var-
ious regions of the central nervous system, where they are
not likely to be targeted by these noxious stimuli, suggests
the existence of endovanilloids. Three different classes of
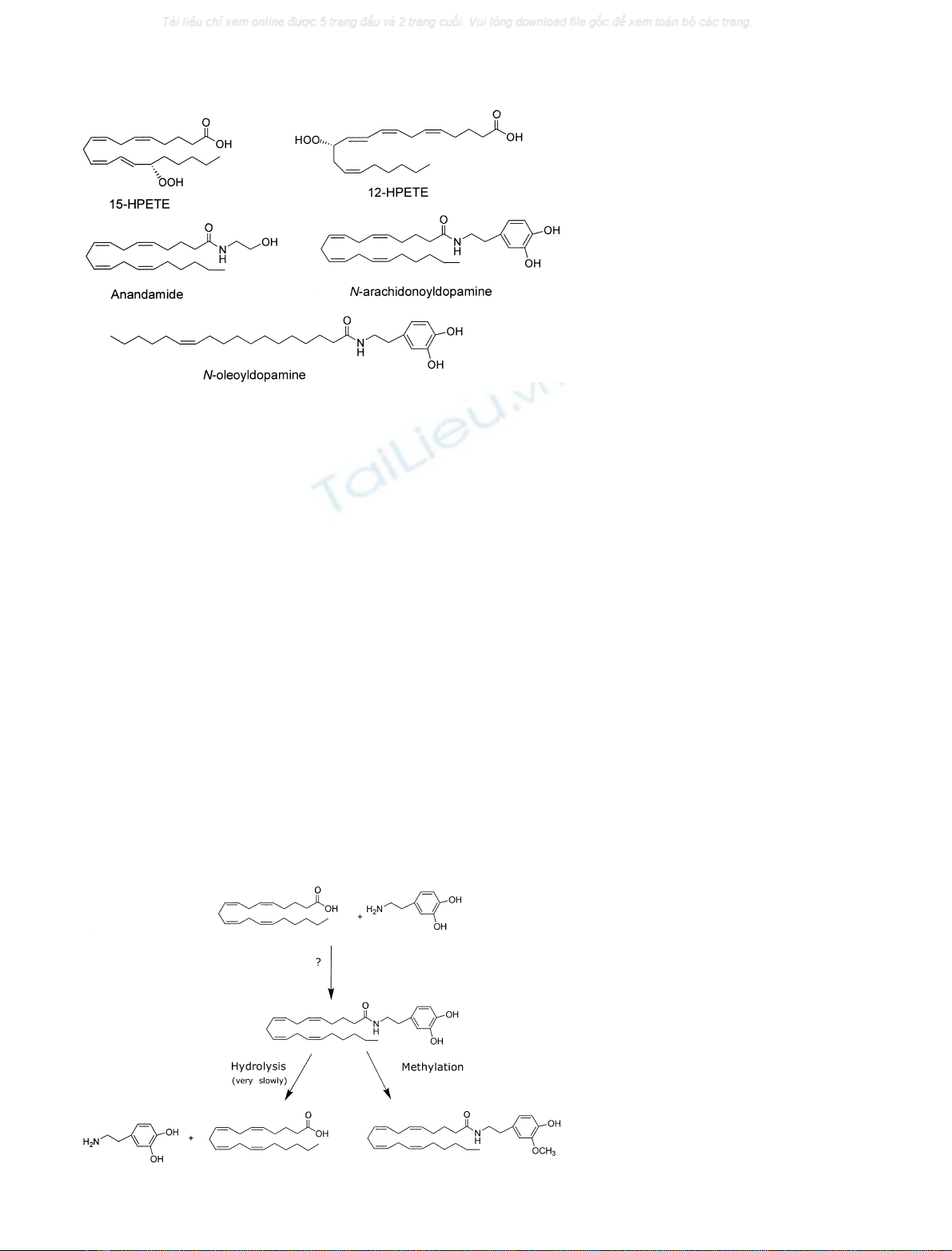
endogenous lipids have been found recently that can
activate TRPV1, i.e. unsaturated N-acyldopamines, lip-
oxygenase products of arachidonic acid and the endo-
cannabinoid anandamide with some of its congeners. To
classify a molecule as an endovanilloid, the compound
should be formed or released in an activity-dependent
manner in sufficient amounts to evoke a TRPV1-mediated
response by direct activation of the channel. To control
TRPV1 signaling, endovanilloids should be inactivated
within a short time-span. In this review, we will discuss,
for each of the proposed endogenous ligands of TRPV1,
their ability to act as endovanilloids in light of the criteria
mentioned above.
Keywords: anandamide; arachidonic acid; cannabinoid;
lipid; signaling; TRP; VR1.
Introduction
The transient receptor potential vanilloid type 1 (TRPV1)
protein is a nonselective cation channel that belongs to the
large family of TRP ion channels and is highly expressed in
small diameter primary afferent fibers [1]. It is a molecular
integrator of noxious stimuli, such as heat and low pH,
and can also be activated by the pungent ingredient of hot
chilli peppers, capsaicin, as well as by other plant toxins, the
most potent of which is resiniferatoxin (RTX) [2]. In
primary sensory neurons, TRPV1 is essential for the
development of inflammatory hyperalgesia [3,4]. Although
initially controversial, it has now been firmly established
that TRPV1 is synthesized in cells outside the peripheral
nervous system, such as keratinocytes, epithial and endo-
thelial cells, where it serves as yet undefined purposes [5].
Furthermore, TRPV1 has also been found in various brain
areas, including dopaminergic neurons of the substantia
nigra, hippocampal pyramidal neurons, hypothalamic neu-
rons, the locus coeruleus in the brainstem and in various
layers of the cortex, where it might be involved in
modulation of synaptic plasticity [6,7].
In the central nervous system or under normal physio-
logical conditions, TRPV1 is unlikely to be activated by
heat or low pH, therefore it has been suggested that other
endogenous ligands of this ion channel exist. Indeed, three
different classes of lipids, all derived from the metabolism
of arachidonic acid (AA), have been recently characterized
that can activate TRPV1, i.e. in chronological order, the
endocannabinoid anandamide, some lipoxygenase products
of AA, and N-arachidonoyldopamine (Fig. 1). To qualify as
an endovanilloid, i.e. an endogenous activator of TRPV1,
the compound should be formed by cells and be released in
an activity-dependent manner in sufficient amounts to evoke
a TRPV1-mediated response by direct binding and subse-
quent activation of the channel. Finally, endovanilloid
signalling should be terminated within a short time-span to
allow a strict regulation of its actions. Therefore, biosyn-
thetic and metabolic pathways for a putative endovanilloid
should be present in close proximity to TRPV1. As the
putative binding site for an endogenous ligand at TRPV1
is intracellular [8,9], it might be expected that putative
endogenous ligands are produced inside the cell or, in the
case of production at a more distant site, brought into the
cell. In this review we will discuss the capabilities of
Correspondence to V. Di Marzo, Instituto di Chimica Biomolecolare,
Consiglio Nazionale delle Ricerche, Via Campi Flegrei 34,
Comprensorio Olivetti, Bldg. 70 80078 Pozzuoli (NA), Italy.
Fax: + 39 081 8041770, Tel.: + 39 081 8675093,
E-mail: vdimarzo@icmib.na.cnr.it
Abbreviations: 12-HETE, 12-hydroxyeicosatetraenoic acid; 12S-and
15S-HPETE, 12-(S)- and 15-(S)-hydroperoxyeicosatetraenoic acid;
AA, arachidonic acid; DRG, dorsal root ganglia; RTX, resinifera-
toxin; TRPV1, transient receptor potential vanilloid type 1 protein.
Note added during revision: During the revision process of this article,
NADA was reported to exert a potent vasodilation of rat mesenteric
arteries via mechanisms including TRPV1 receptors as well as cann-
abinoid CB1 receptors and an as-yet-uncharacterized cannabinoid
endothelial receptor [O’Sullivan, S.E., Kendall, D.A. & Randall, M.D.
(2004). Br. J. Pharmacol.,141, 803–812].
(Received 16 January 2004, revised 13 February 2004,
accepted 18 February 2004)
Eur. J. Biochem. 271, 1827–1834 (2004) ÓFEBS 2004 doi:10.1111/j.1432-1033.2004.04081.x
N-arachidonoyldopamine, lipoxygenase products of AA
and anandamide to act as endogenous activators for TRPV1
in vivo in light of the above mentioned criteria.
N
-arachidonoyldopamine
Biosynthesis
N-arachidonoyldopamine was characterized originally in
the striatum of bovine brain and its distribution in the rat
nervous system is as follows (greatest abundance to lowest):
striatum > hippocampus > cerebellum > thalamus >>
dorsal root ganglia (DRG) [10]. The basal levels of
N-arachidonoyldopamine are low (6 pmolÆg
)1
striatum)
and its stimulus-evoked formation has yet to be demon-
strated [10]. Noteworthy, other congeners of this compound
have been detected recently in the bovine brain, i.e.
N-oleoyldopamine, N-palmitoyldopamine and N-stearoyl-
dopamine [11]. Of these, N-oleoyldopamine also possesses
the ability to activate TRPV1 with the same potency as
N-arachidonoyldopamine [11]. N-arachidonoyldopamine
can theoretically be formed through two biosynthetic
pathways [10]. Firstly, a direct condensation of AA (or its
Coenzyme A derivative) with dopamine has been proposed,
but the enzyme responsible for this condensation has not
yet been characterized. The second route is an indirect
pathway in which N-arachidonoyltyrosine is converted
into N-arachidonoyldopamine. Interestingly, TRPV1
mRNA was colocalized with mRNA of tyrosine hydroxy-
lase in dopaminergic neurons of the substantia nigra,
which project to the striatum [6]. To date, conclusive
experimental evidence for either pathway is still lacking.
If N-arachidonoyldopamine acts in a paracrine manner,
it has to be transported into the cell to reach its site of
action. In support to this hypothesis, N-arachidonoyl-
dopamine was rapidly taken-up by C6 glioma cells via the
rapid facilitated diffusion process [10] that is also respon-
sible for anandamide transport across membranes (reviewed
in [12]).
Degradation
The same membrane transport process carrying N-arachi-
donoyldopamine into the cell can theoretically also be used
to extrude it from the cell, thereby terminating its actions
at TRPV1. Other possible inactivation pathways are the
hydrolysis of its amide bond by a hydrolase or its
methylation of the hydroxyl group of its cathechol moiety
by a cathechol-O-methyl-transferase (Fig. 2). The latter
route might represent the inactivation pathway in in vivo
nervous tissues where this enzyme is abundant because
N-arachidonoyldopamine was only very slowly hydrolysed
by brain homogenates and its methylated derivative was
less potent or inactive at TRPV1 [10].
Fig. 1. Chemical structures of endogenous
TRPV1 ligands.
Fig. 2. Metabolic pathways of N-arachido-
noyldopamine.
1828 M. van der Stelt and V. Di Marzo (Eur. J. Biochem. 271)ÓFEBS 2004
Pharmacology and physiological actions
N-arachidonoyldopamine is a full agonist of TRPV1 and
the most potent endogenous lipid ligand discovered to date.
It has a K
d
of approximately 5–10 l
M
in binding assays
performed in heterologous or native expression systems,
respectively [13,14]. It is five to 10-fold more potent than
anandamide and almost equipotent as capsaicin in some
functional assays, with an EC
50
in Ca
2+
-influx assays of
approximately 50 n
M
at human and rat TRPV1 over-
expressed in HEK293 cells. N-arachidonoyldopamine is
also able to rapidly desensitize the TRPV1 receptor to
subsequent capsaicin activation [10]. Its potency seems to be
dependent on the cell and assay conditions used to assess its
functional activity, because in rat DRG neurons (using
Ca
2+
imaging) and rat TRPV1-CHO cells (using a Ca
2+
-
uptake assay) its potency is in the low micromolar range
(0.8–5 l
M
) [10,13]. Exogenous N-arachidonoyldopamine
induces, in a TRPV1-dependent manner, calcitonin gene-
related peptide and substance P release from spinal cord
slices and enhances hippocampal paired-pulse depression
(EC
50
0.1–0.2 l
M
[10]). It is also able to induce TRPV1-
mediated thermal hyperalgesia [10], and constriction of
isolated bronchi and urinary bladder [15], albeit, in these
two assays N-arachidonoyldopamine is much less potent
and efficacious than capsaicin. Species differences and
pharmacodynamic factors (such as reduced bioavailability
and limited access to the intracellular site on TRPV1) were
suggested to be responsible for these varying potencies [15].
As yet, no TRPV1-mediated physiological or pathological
conditions have been attributed to endogenously formed
N-arachidonoyldopamine.
Lipoxygenase products of arachidonic acid
Biosynthesis
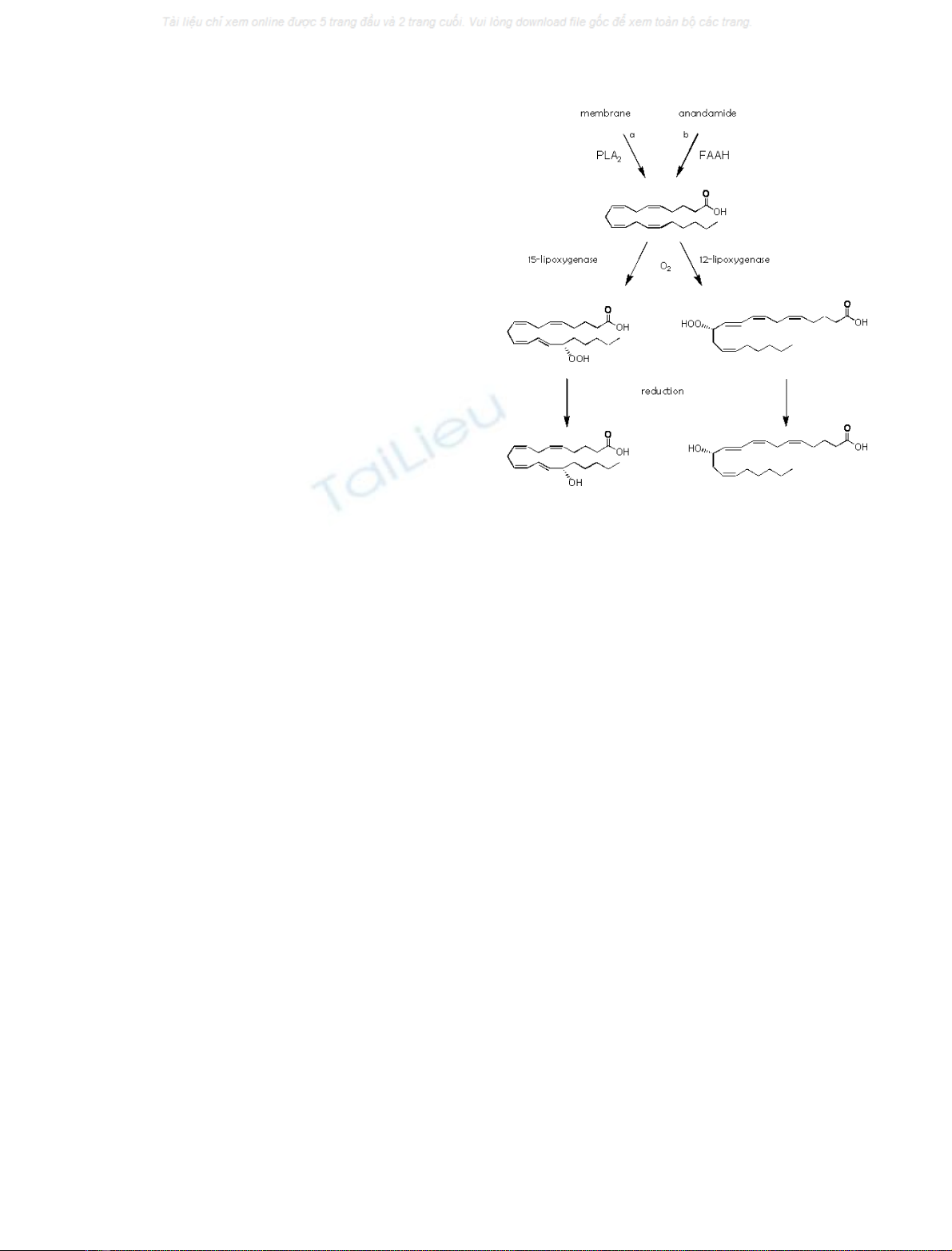
Various oxygenated AA derivatives were shown to activate
TRPV1 [16]. 12-(S)- and 15-(S)-hydroperoxyeicosatetrae-
noic acid (12S-and15S-HPETE) possessed the highest
potency. These AA metabolites can be produced by
lipoxygenases that introduce molecular oxygen in a regio-
and stereoselective dependent manner in AA released from
the membrane by phospholipase A
2
enzymes (Fig. 3) [17].
Several isoforms for both 12S-and15S-lipoxygenases have
been identified. 15(S)-Lipoxygenase-1 has been found in
human eosinophils and a second isoform, 15(S)-lipoxyge-
nase-2, is expressed in the prostate, lung, cornea and hair
roots [18]. There are three 12-lipoxygenase isoforms that
have a broad tissue distribution, including the lung, pineal
gland, spleen, macrophages and keratinocytes [19]. 12S-
Lipoxygenase has also been found in the canine brain,
including basal ganglia, hippocampus and hypothalamus
[20,21]. Importantly, 12-lipoxygenase mRNA was found
to be coexpressed with TRPV1 in rat DRG neurons [22].
However, colocalization with TRPV1 in neurons of the
central nervous system has not been studied yet.
Lipoxygenases are not stimulated by calcium, but their
substrate AA can be liberated from the membrane by an
agonist-stimulated increase in intracellular calcium that
activates Ca
2+
-dependent phospholipases A
2
[17]. For
example, it was shown that the potent inflammatory
mediator bradykinin could induce the production of
12-hydroxyeicosatetraenoic acid (12-HETE), a metabolic
product of 12-HPETE, from rat primary sensory neurons
[22]. Furthermore, AA can be produced from anandamide
by hydrolysis of its amide bond by fatty acid amide
hydrolase [23]. Interestingly, 12/15-lipoxygenases can also
accept AA esterified into membrane lipids and the endo-
cannabinoid anandamide as a substrate [18,24,25–27]. The
lipoxygenase products of anandamide are potent endo-
genous inhibitors of fatty acid amide hydrolase [27,28]
and might stimulate TRPV1 themselves [29,30], although
attempts to demonstrate their direct activity at these
receptors have given negative results [8,31].
Degradation
The hydroperoxy group in the lipoxygenase products of AA
is very labile and easily reduced into a hydroxy-group by
glutathione peroxidases, which might constitute an inacti-
vation pathway, because the reduced lipoxygenase products
are far less potent on TRPV1 (Fig. 3) [16].
Pharmacology and physiological actions
12-HPETE could displace [
3
H]RTX from recombinant
TRPV1-HEK293 cells more potently than capsaicin
(K
i
¼0.35 and 2.5 l
M
, respectively) [22]. However, in
inside-out patches of neonatal rat dorsal root ganglia
neurons 12(S)- and 15(S)HPETE had an EC
50
values of
approximately 8 l
M
, which was seven- to eightfold higher
than that for capsaicin as measured by single channel
current recordings [16]. To date, no pharmacological study
has described the activity of exogenous lipoxygenase
products on typical TRPV1-mediated responses in vivo or
Fig. 3. Biosynthetic and metabolic pathways of 12- and 15-HPETE.
Pathways indicated (a) under physiological conditions and during
pharmacological assays and (b) during pharmacological assays.
ÓFEBS 2004 Endovanilloids (Eur. J. Biochem. 271) 1829
ex vivo, such as hyperalgesia, and vasodilation or broncho-
or urinary bladder constriction. This lack of data might
be due to practical problems such as the instability of
these compounds. However, some studies indirectly suggest
that endogenously produced lipoxygenase metabolites
are responsible for TRPV1-mediated actions in vivo.For
example, protease-activated receptor-2 activation causes
endothelium-dependent coronary vasodilation in a capsaze-
pine-sensitive manner, which might be mediated through a
lipoxygenase metabolite, because three structurally different
lipoxygenase inhibitors were able to attenuate this response
[32]. In their elegant study, Shin et al. provided evidence
that 12(S)-HPETE is produced endogenously in sensory
neurons upon stimulation of sensory nerve endings by the
inflammatory mediator bradykinin, and activates TRPV1.
This might suggest that this lipoxygenase product is
important in the development of inflammatory pain [22].
As TPRV1 activation in bronchial afferents leads to
bronchoconstriction and lipoxygenase activity is up-regula-
ted during inflammatory reactions, it has also been hypo-
thesized that lipoxygenase product-mediated activation of
TRPV1 might contribute to bradykinin-induced hypersen-
sitivity of airways and asthma [33]. Subsequent studies
performed with TRPV1 null mice have shown, however,
that whereas TRPV1 may have a modulatory role in the
activation of bronchopulmonary C-fibres induced by brady-
kinin, it is not required for action potential discharge evoked
by this stimulus [34].
Anandamide
Biosynthesis
The endogenous lipid anandamide was isolated from pig
brain in 1992 [35] and characterized originally as an
endogenous agonist of cannabinoid receptors. Therefore,
its biosynthetic and metabolic pathways have been studied
in great detail (Fig. 4) [36,37]. In 1999, it was reported that
anandamide could also activate rat TRPV1 receptors in
mesenteric arteries as well as both rat and human TRPV1 in
heterologous expression systems. This made this compound
the first discovered endogenous ligand for TRPV1 [38,39]. It
is widely recognized that anandamide is not stored in
vescicles like other mediators but by analogy with other
eicosanoids, is produced on demandin a Ca
2+
-dependent
manner [40]. This is the result of a biosynthetic mechanism
relying on the existence of a phospholipid precursor for
anandamide, and of a Ca
2+
-sensitive phosphodiesterease
for the conversion of this precursor into anandamide.
Although the biosynthetic route underlying the formation
of anandamide has been extensively studied, the phospho-
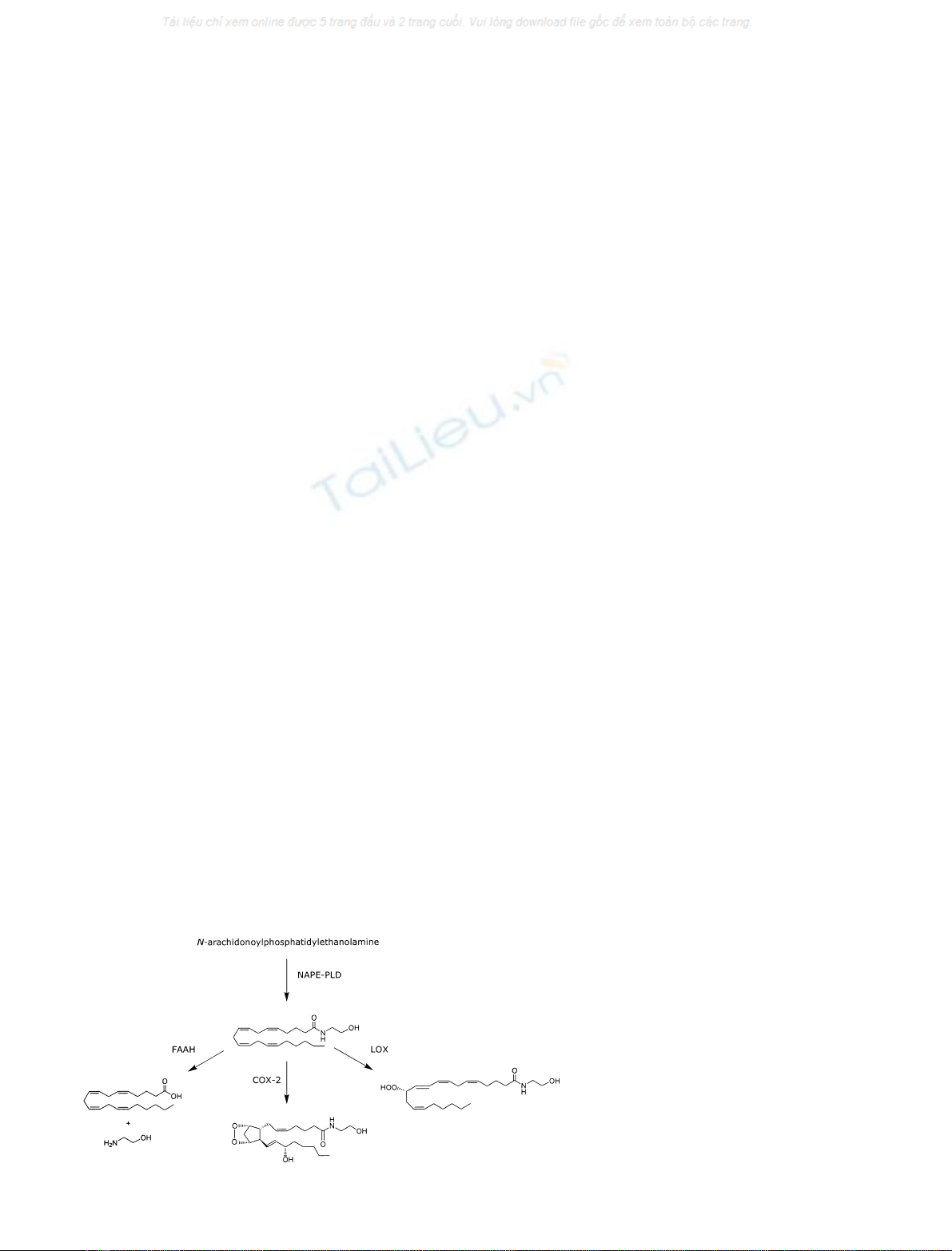
lipase D (PLD) responsible for release of anandamide from
its precursor N-arachidonoylphosphatidylethanolamine has
only been purified recently, cloned and characterized [41].
It is still unknown which type of neurons express the
biosynthetic enzymes, and therefore a classification of
anandamidergicneurons is not yet possible. Nevertheless,
anandamide has been found in all the brain regions which
express TRPV1, with high levels in hippocampus, substantia
nigra and striatum. Sensory neurons of rat dorsal root
ganglia are also able to produce anandamide in high
amounts [42].
Degradation
Theoretically, intracellular anandamide can be inactivated
through two concurrent processes [43]. Firstly, anandamide
might be extruded from the cell via a selective transporter.
This might be the same protein that is responsible for the
uptake of anandamide from the extracellular space into the
cell, because, among other things, anandamide has been
proposed to be transported by a carrier-facilitated diffusion
process according to its concentration gradient across the
membrane [44,45]. This process might be bi-directional,
because it is neither dependent on external Na
+
nor affected
by metabolic inhibitors. Anandamide uptake (and possibly
its release) can be stimulated by NO [28] and blocked by
selective inhibitors [46–48]. However, the elusive nature of
the putative protein responsible for endocannabinoid
transport across membranes has initiated a debate on its
existence [12].
Secondly, once inside the cell, anandamide undergoes
metabolism via two possible pathways: hydrolysis and
oxygenation [43,49,50]. The amide bond in anandamide
can be hydrolysed, which yields AA and ethanolamine.
Although AA does not activate TRPV1 by itself, it is a
substrate for lipoxygenases, which can produce metabolites
active on this channel (see above). Therefore, when studying
the effects of exogenous anandamide at TRPV1 its hydro-
lysis should be taken into account. Fatty acid amide
hydrolase, the protein responsible for anandamide hydro-
lysis in vivo, has been cloned and studied in detail [23,51]. It
is an intracellular membrane-associated hydrolase with high
activity in several brain areas, including the hippocampus,
Fig. 4. Biosynthetic and metabolic pathways of
anandamide.
1830 M. van der Stelt and V. Di Marzo (Eur. J. Biochem. 271)ÓFEBS 2004

subtantia nigra and striatum [52]. At the moment it is
not known whether TRPV1 is coexpressed with fatty acid
amide hydrolase in the same neurons.
Much less is known about the oxygenation pathways.
Lipoxygenase- and cycloxygenase-catalyzed oxygenation of
anandamide has been shown to generate a vast array of
possibly biologically active compounds, such as hydro-
peroxy-anandamides and prostamides [50]. While the latter
compounds are not able to activate TRPV1, and hence
conversion of anandamide by cyclooxygenase-2 represents
an inactivation pathway, the lipoxygenase products of
anandamide might still be able to activate this channel
[29,30], much in the same way they appear to be still active
to some extent also on cannabinoid receptors [27].
Pharmacology and physiological actions
The pharmacology of anandamide actions on TRPV1 has
recently been reviewed extensively by Ross [53]. In short,
anandamide displaces [
3
H]RTX from TPRV1 with a K
i
of
2l
M
in recombinant cell lines, which is similar to that of
capsaicin [54,55]. However, its potency in various assays is
usually five- to 10-fold lower than that of capsaicin. For
example, in high recombinant expression systems, the EC
50
value for anandamide-induced Ca
2+
-influx ranges from 0.4
to 5 l
M
and anandamide appears to act as a full agonist,
while in native systems, such as Ca
2+
-influx and inward
current in DRG neurons, anandamide is a partial agonist
with a potency varying from 6 to 10 l
M
[10,16,56]. In
isolated organs, exogenous anandamide has also been
shown to induce typical TRPV1-mediated effects with
varying potency and efficacy. For example, anandamide
induces CGRP-mediated relaxation of blood vessels with an
EC
50
of 0.3–0.8 l
M
as a full agonist [38,57,58], while it is
much less potent when causing tachykinin-mediated con-
striction of bronchi and urinary bladder (EC
50
varying from
6to>10l
M
)[57].
Due to its low potency and partial agonism in some
assays, anandamide’s ability to be a physiologically relevant
activator of TRPV1 was originally controversial [59].
However, it is now well established that the potency and
efficacy of (exogenous) anandamide at TRPV1 receptors are
influenced by a multitude of different factors, ranging from
assay conditions and species differences to TRPV1 modi-
fication and the ability of anandamide to reach the
intracellular binding site on TRPV1.
Thus, due to its low intrinsic efficacy, anandamide is a
partial agonist in tissues with a low receptor reserve (e.g.
bronchi), but it appears to be a full agonist in tissues with a
high receptor reserve such as the mesentery artery [53,57]. In
several pathological conditions, TRPV1 activity/expression
is upregulated, and this results in a significantly higher
efficacy of anandamide [60]. It has been shown that ethanol,
which is frequently used as a vehicle to dissolve anand-
amide, can potentiate TRPV1 mediated-responses to
anandamide via an unknown mechanism [61], whereas
bovine serum albumin and plastic may prevent anandamide
from reaching the intracellular binding site [55]. Apart from
high temperature (> 43 °C) and low pH (< 7.2), which
may activate and/or sensitize TRPV1, multiple signalling
pathways have also been shown to interact with TRPV1 to
modify its gating properties and response to anandamide
[1]. The channel might be sensitized by (a) removal of its
inhibition by phosphatidylinositolbisphosphate, via PLC-
mediated hydrolysis [62], (b) protein kinase C-catalysed
phosphorylation following PLC-mediated diacylglycerol
release [63,64], (c) protein kinase A-mediated phosphoryla-
tion [31], (d) phosphorylation induced by by calmodulin-
dependent protein kinase II [65] or (e) voltage-dependent
priming [66]. Conversely, TRPV1 can be rapidly desensi-
tized subsequent to activating stimuli by a calmodulin-
dependent step [67].
As discussed in the previous paragraph, the level of
expression and the activity of the putative anandamide
transporter, which may vary in different types of cells, is of
crucial importance for exogenous anandamide to reach its
cytosolic binding site at TRPV1 [8,57]. The rapid metabo-
lism of anandamide inside cells may also limit its activation
of TRPV1 [8,54,68], or potentiate its effect in the case of
lipoxygenase-mediated conversion [29,30].
N-acyl-ethanolamine anandamide congeners, which are
cobiosynthesized with anandamide from the phospholipase
D-dependent hydrolysis of the corresponding N-acyl-
phosphatidylethanolamines [41], also significantly enhance
ananadamide effects at TRPV1 [55,69]. One of these
compounds, N-oleoylethanolamine, was even found to be
able to activate TRPV1 per se under certain conditions
[70]. Last, but not least, anandamide functional activity at
TRPV1 can be significantly masked by its concomitant
activity on cannabinoid CB
1
receptors, particularly in those
tissues and cells where the two receptors are coexpressed
and are coupled to opposing biological effects [80]. In this
case, a significant enhancement of the potency of anand-
amide at TRPV1 can be observed in the presence of CB
1
antagonists [80].
At the moment, very few studies have been reported in
which endogenous anandamide was shown to activate
TRPV1 in vivo. Although exogenous anandamide induces
TRPV1-mediated vasodilation, calcium influx in DRG
neurons and bronchoconstriction in situ, which might
suggest that endogenous anandamide is involved through
TRPV1 in physiological processes such as blood pressure,
pain sensation and airway responsiveness, respectively, to
date there is only little experimental data providing conclu-
sive evidence supporting these hypotheses [14,60,71,72].
However, recently the first example of activation of TRPV1
by endogenous anandamide has been reported. Ananda-
mide, endogenously produced in the inflamed ileum of rats
treated with toxin A, was shown to cause TRPV1-depend-
ent ileitis [68]. This finding strengthens the hypothesis that
anandamide behaves as an endovanilloid particularly under
some pathological conditions, such as inflammation.
With respect to the central nervous system, it is not
known whether endogenously formed anandamide activates
TRPV1 under normal physiological conditions. Exogenous
anandamide induces, in hippocampal slices, a TRPV1-
mediated enhancement of paired-pulse depression, which is
a form of short-term synaptic plasticity [73]. Tonically
activated TRPV1 receptors have been found in the
substantia nigra compacta. While the mechanism of activa-
tion has not been elucidated, this suggests that TRPV1
might be involved in the control of movement [74].
Interestingly, the anandamide uptake inhibitor and
TRPV1 agonist, AM404 [N-(4-hydroxyphenyl)-arachidonyl-
ÓFEBS 2004 Endovanilloids (Eur. J. Biochem. 271) 1831




















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)




