
The role of helix 8 and of the cytosolic C-termini in
the internalization and signal transduction of B
1
and B
2
bradykinin receptors
Alexander Faussner
1
, Alexandra Bauer
1
, Irina Kalatskaya
1
, Steffen Schu
¨ssler
1
, Cornelia Seidl
1
,
David Proud
2
and Marianne Jochum
1
1 Ludwig-Maximilians-Universita
¨t, Abteilung fu
¨r Klinische Chemie und Klinische Biochemie, Mu
¨nchen, Germany
2 Department of Physiology & Biophysics, University of Calgary, Alberta, Canada
G protein-coupled receptors (GPCRs) form a vast and
diverse superfamily of proteins with seven transmem-
brane-spanning domains. They transduce specific exter-
nal stimuli to intracellular second messenger-dependent
effector cascades via recruitment and activation of
heterotrimeric G proteins [1]. To protect cells from
chronic overstimulation, desensitization processes such
as the rapid attenuation of receptor responsiveness and
Keywords
G protein-coupled receptor, helix 8
modeling, internalization, receptor chimera
Correspondence
A. Faussner, Ludwig-Maximilians-
Universitaet Muenchen, Abt. Klinische
Chemie und Klinische Biochemie,
Nussbaumstr. 20, D-80336 Muenchen,
Germany
Tel: +49 89 51602602
Fax: +49 89 51604740
E-mail: alexander.faussner@
med.uni-muenchen.de
(Received 21 July 2004, revised 14 September
2004, accepted 15 September 2004)
doi:10.1111/j.1432-1033.2004.04390.x
Determinants for desensitization and sequestration of G protein-coupled
receptors often contain serine or threonine residues located in their C-ter-
mini. The sequence context, however, in which these residues have to
appear, and the receptor specificity of these motifs are largely unknown.
Mutagenesis studies with the B
2
bradykinin receptor (B
2
wt), stably
expressed in HEK 293 cells, identified a sequence distal to N338
(NSMGTLRTSI, including I347 but not the basally phosphorylated S348)
and in particular the TSI sequence therein, as a major determinant for
rapid agonist-inducible internalization and the prevention of receptor
hypersensitivity. Chimeras of the noninternalizing B
1
bradykinin receptor
(B
1
wt) containing these B
2
wt sequences sequestered poorly, however, sug-
gesting that additional motifs more proximal to N338 are required. In fact,
further substitution of the B
1
wt C-terminus with corresponding B
2
wt
regions either at C330(7.71) following putative helix 8 (B
1
CB
2
) or at the
preceding Y312(7.53) in the NPXXY sequence (B
1
YB
2
) resulted in chi-
meras displaying rapid internalization. Intriguingly, however, exchange
performed at K322(7.63) within putative helix 8 generated a slowly inter-
nalizing chimera (B
1
KB
2
). Detailed mutagenesis analysis generating addi-
tional chimeras identified the change of V323 in B
1
wt to serine (as in B
2
wt)
as being responsible for this effect. The slowly internalizing chimera as well
as a B
1
wt point-mutant V323S displayed significantly reduced inositol
phosphate accumulation as compared to B
1
wt or the other chimeras. The
slow internalization of B
1
KB
2
was also accompanied by a lack of agonist-
induced phosphorylation, that in contrast was observed for B
1
YB
2
and
B
1
CB
2
, suggesting that putative helix 8 is either directly or indirectly
(e.g. via G protein activation) involved in the interaction between the
receptor and receptor kinases.
Abbreviations
BK, bradykinin; B
x
wt, wild-type B
x
bradykinin receptor; DAK, desArg10kallidin; GPCR, G protein-coupled receptor; GRK, G protein-coupled
receptor kinase; HEK, human embryonic kidney; IP, inositol phosphate.
FEBS Journal 272 (2005) 129–140 ª2004 FEBS 129

G protein uncoupling are essential. Some of these
desensitization mechanisms involve the translocation
of the stimulated receptor to distinct compartments
and endocytosis after phosphorylation of serine ⁄threo-
nine residues mostly located in the receptor C-termini
(reviewed in [2]). Little is known so far about the
sequence context in which these residues have to
appear to become phosphorylated by kinases and to
be recognized by the internalization machinery. In par-
ticular, the receptor specificity of these motifs is not
completely understood.
The B
1
bradykinin receptor (B
1
wt) is one of the
few receptors belonging to the class A family of rho-
dopsin-like ⁄b2-adrenergic-like GPCRs that does not
get internalized, i.e. sequestered to intracellular com-
partments upon agonist stimulation [3]. It does, how-
ever, respond with translocation to caveolae but these
remain essentially on the cell surface [4,5]. No phos-
phorylation of B
1
wt either under basal conditions or
after stimulation has been detected [6]. The B
2
brady-
kinin receptor (B
2
wt), by contrast, is a more typical
GPCR that gets internalized rapidly following activa-
tion. Phosphorylation of several serine ⁄threonine resi-
dues in the C-terminus of this receptor, and the
importance of these events for receptor sequestration,
have been described in detail [7]. Whether coupling of
b-arrestin(s) then follows this, and whether internal-
ization occurs via clathrin-coated pits, caveolae or
other less well-defined mechanisms is still a topic of
debate [5,7,8].
The two bradykinin (BK) receptor subtypes exhibit
a relatively low overall amino acid identity of about
36% [9,10], most of it located in the transmem-
brane regions. Both receptors stimulate phospholipase
C
b
-mediated inositol phosphate (IP) release leading to
an elevation of intracellular [Ca
2+
] levels, primarily via
coupling to G protein G
q⁄11
[3,10,11].
They become activated by the kinins, small pro-
inflammatory peptides with great vasoactive potential
implicated as mediators of inflammation, pain and
hyperalgesia [12,13]. The nonapeptide BK and Lys-BK
(kallidin) bind with high affinity to B
2
wt but not B
1
wt.
Removal of the C-terminal arginine through carboxy-
peptidases generates desArg9-bradykinin and desArg10-
kallidin (DAK), two peptides that now bind exclusively
to the B
1
wt [14].
In this study we wanted to exploit the fact that the
B
1
wt does not internalize as part of a gain-of-function
approach to provide insight into the receptor speci-
ficity of the B
2
wt internalization motif. The resulting
data also hint at a receptor specific role of the putative
helix 8 in G protein activation and interaction with
receptor kinases.
Results
Construction of truncated and point mutated
B
2
wts and B
1
⁄B
2
receptor chimeras
Several studies with truncations of, and deletions in,
the C-terminal part of B
2
wt have demonstrated that
this part plays a central role in the internalization of
this receptor [7,15,16]. A similar function of the C-ter-
minus was also observed in other GPCRs with short
third intracellular loops [2]. In particular, several serine
or threonine residues that become phosphorylated by
protein kinase C and ⁄or by GPCR kinases (GRKs)
following receptor activation are absolutely required
for rapid B
2
wt sequestration [17].
To determine the C-terminal sequence(s) of the B
2
wt
minimally required for internalization we created two
new B
2
wt truncations, I347* and N338* (Fig. 1). The
former removed the C-terminus including residue
S348, which has been shown to be responsible for the
basal phosphorylation of the B
2
wt, while the latter
truncation deleted all serine and threonine residues
(S339, T342, T345, S346) shown to be phosphorylated
following stimulation of the receptor [17]. In addition,
a triple alanine replacement of T345-S346-I347(S348)
(mutated residues are underlined) was made, as this
sequence strongly resembles the C-terminal STLS-
motif in the AT
1A
angiotensin II receptor, where a tri-
ple alanine substitution of STL almost completely
abolished receptor sequestration [18].
All of these B
2
receptor constructs were highly
expressed (Table 1). We took care therefore to use
[
3
H]BK concentrations below 1.5 nm, as we have
shown that receptor internalization rates are independ-
ent of agonist concentration in this range [19]. The
truncation I347* internalized as rapidly as B
2
wt
(Fig. 2A) demonstrating that the distal C-terminus,
and in particular S348 and its basal phosphorylation,
do not play a decisive role in the sequestration process.
This notion was further supported by results obtained
with a point mutation of S348 to alanine that exhibited
an almost identical internalization rate as the B
2
wt ([7]
and data not shown).
In contrast, deletion of all phosphorylation sites in
N338* led to an extremely diminished [
3
H]BK internal-
ization (Fig. 2A). Indeed, even the internalization cal-
culated for each time point is an overestimate because
a shift to lower affinity at 37 C by the receptors
remaining on the cell surface can be assumed, as there
was a clear drop in surface binding that could not be
accounted for by the amount of internalized agonist
[20]. As the internalization is expressed in percentage
of total binding, decreasing the binding affinity of the
Role of helix 8 and C-termini in bradykinin receptors A. Faussner et al.
130 FEBS Journal 272 (2005) 129–140 ª2004 FEBS
surface receptors simulates an apparent increase in
internalization over time. Although it internalized
[
3
H]BK much slower than the B
2
wt, N338* neverthe-
less was able to induce an accumulation of total IPs
identical to that observed for the B
2
wt (Table 1). This
truncated receptor even became hypersensitive, as its
EC
50
for the IP response was 10-fold lower than
that of B
2
wt (0.072 ± 0.038 nmvs. 0.79 ± 0.34 nm;
Table 1). Most interestingly, the effects of a truncation
at N338 could also be achieved in part by the triple
mutation TSIfiAAA as this construct displayed sim-
ilar properties to truncation N338*. It exhibited a
markedly reduced capacity to internalize [
3
H]BK albeit
not as diminished as truncation N338* and was at
least as hypersensitive with an EC
50
¼0.058 ±
0.06 nm(Table 1). This sequence obviously contributes
significantly to agonist internalization and signaling of
B
2
wt.
However, transfer of the B
2
wt C-terminus starting
with this sequence, to the C-terminus of the intact
noninternalizing B
1
wt (B
1
RB
2
; Fig. 1), conferred very
little capability to internalize its agonist to the B
1
wt
(Fig. 2B). The chimera B
1
NB
2
containing all serine
and threonine residues critical for B
2
wt sequestration,
in contrast, was able to internalize [
3
H]DAK at a rate
approximately half of the maximal rate (40% after
10 min) seen for the B
2
wt with [
3
H]BK (Fig. 2B).
As it was obviously not sufficient to simply add the
B
2
wt phosphorylation sites to the B
1
wt to gain full
receptor sequestration as observed in the B
2
wt, we fur-
ther substituted the C-termini of the B
2
wt into the
B
1
wt at two residues conserved in both receptor sub-
types (Fig. 1); specifically at the conserved cysteine
[Cys330(7.71) in B
1
wt, Cys324(7.72) in B
2
wt] that in
the B
2
wt is palmitoylated (chimera B
1
CB
2
) and at
Y7.53 within the NPXXY sequence (chimera B
1
YB
2
)
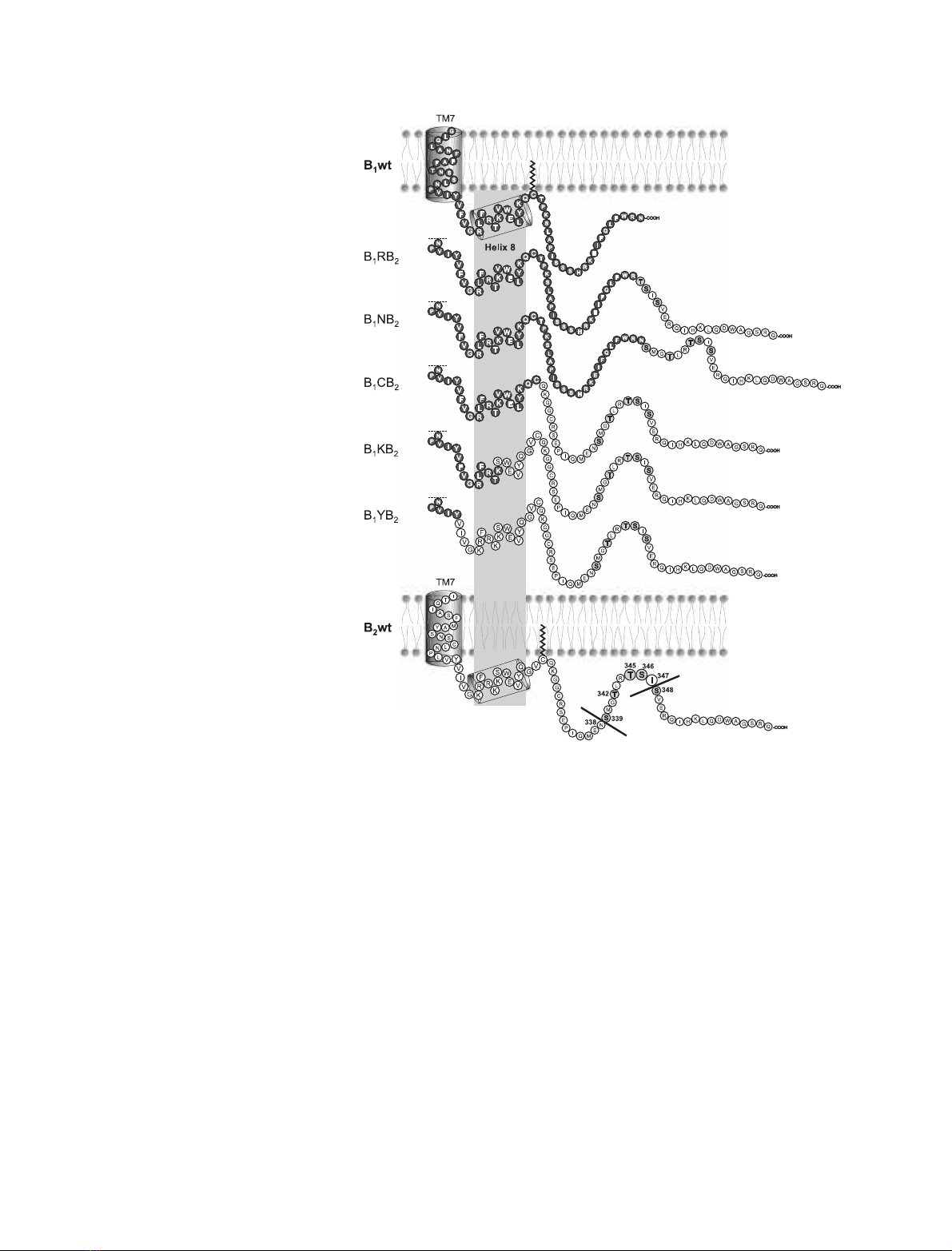
Fig. 1. Schematic representation of the
C-terminal B
1
wt and B
2
wt sequences and
chimera thereof. The C-terminal sequences
beginning at transmembrane domain 7 are
shown. B
1
wt parts are indicated in filled
circles, B
2
wt portions in unfilled ones. The
phosphorylation sites in B
2
wt are highlighted
in light grey, and the position number is indi-
cated. The grey box outside the membrane
indicates the region of the putative cytosolic
helix 8 as found in the crystal structure of
bovine rhodopsin [24]. The assumed palmi-
toylation of B
1
wt and B
2
wt is indicated.
A. Faussner et al.Role of helix 8 and C-termini in bradykinin receptors
FEBS Journal 272 (2005) 129–140 ª2004 FEBS 131

at the end of the seventh transmembrane domain. We
have shown previously that a B
1
CB
2
chimera stably
expressed in Chinese hamster ovary cells was seques-
tered rapidly upon activation [16]. This was confirmed
in human embryonic kidney (HEK) 293 cells (Fig. 2B).
As the chimera B
1
YB
2
exhibited a slightly attenuated
internalization compared to B
1
CB
2
(Fig. 2B), and the
latter apparently did not gain the full internalization
capability of the B
2
wt, we next tested the possibility
that there is an optimum site for creating rapidly inter-
nalizing chimeras at K7.63 between these two residues
and generated the chimera B
1
KB
2
(Fig. 1). Surpris-
ingly, B
1
KB
2
showed poor ability to internalize
[
3
H]DAK (30% after 10 min), with an internalization
far below those seen for B
1
CB
2
and B
1
YB
2
(Fig. 2B).
Agonist-induced internalization of modified
B
1
KB
2
constructs
The segment between the NPXXY motif and the con-
served cysteine represents one of the regions with the
highest sequence identity between B
1
wt and B
2
wt. The
different internalization of B
1
KB
2
and B
1
CB
2
was
therefore even more surprising given that these two
chimeras have only minor sequence differences
(Fig. 3A). Therefore we considered three possibilities
to explain the cause of this drop in the internalization
of B
1
KB
2
as compared to B
1
CB
2
. First, that the two
residues (KQ) preceding the cysteine were pivotal; sec-
ond, that the cysteine itself needs to be at a specific
position in the C-terminus; or third, that the B
1
residue
V323 instead of the serine is essential in this posi-
tion. Thus, we created three additional chimeras to
test these possibilities: (a) B
1
KB
2
⁄QGVfiKQ; (b)
B
1
KB
2
⁄VCfiCV; and (c) B
1
KB
2
⁄SfiV (Fig. 3A).
Substituting KQ for QGV in B
1
KB
2
led to distinctly
increased agonist internalization as compared with
B
1
KB
2
. This increase was not due to a corrected posi-
tion of the cysteine, as it was not observed with
B
1
KB
2
⁄VCfiCV (Fig. 3B).
A major effect, however, was seen with the change
of the polar serine (back) to the nonpolar valine
(B
1
KB
2
⁄SfiV), the amino acid that is normally found
in this position in the B
1
wt. This replacement led to a
chimera exhibiting rapid internalization (60% after
10 min) that was comparable to that of B
1
CB
2
and
B
1
YB
2
(Fig. 2B).
Phosphorylation patterns of B
2
wt and of B
1
⁄B
2
chimeras reflect their agonist-inducible
internalization
Agonist-induced phosphorylation of serine and threo-
nine residues in the C-terminus has been shown to be
a prerequisite for internalization of B
2
wt and other
receptors [17,21]. B
2
wt in HEK 293 cells displayed a
distinct phosphorylation even in the absence of an
agonist (Fig. 4), as reported recently [22]. When stimu-
lated for 5 min with a saturating concentration of 1 lm
BK at 37 C, however, B
2
wt responded with a marked
increase (2.50 ± 0.15-fold over basal) in phosphoryla-
tion. The chimera on the other hand displayed little
basal phosphorylation in the absence of their agonist
DAK, although this may, in part, be a sensitivity prob-
lem due to their lower expression levels. Nevertheless,
the rapidly internalizing chimeras B
1
YB
2
and B
1
CB
2
Table 1. Receptor density (B
max
), receptor affinity (K
d
), basal and stimulated total IP accumulation, and EC
50
of B
2
wt, B
1
wt and B
1
⁄B
2
receptor chimera. ND, not determined.
Receptor construct
B
maxa
(fmolÆmg protein
)1
)
K
d
(nM)
IP accumulation
EC
50
± SEM
(nM)Unstimulated (30 minÆbasal
)1
)
B
2
wt 10400 ± 600 3.91 ± 1.06 1.93 ± 0.17 12.86 ± 1.37 (n¼7) 0.79 ± 0.34 (n¼4)
I347* 5020 ± 900 3.76 ± 1.61 ND ND 1.13 ± 0.47 (n¼4)
TSIfiAAA 5298 ± 1080 2.82 ± 0.92 2.29 ± 0.77 10.68 ± 2.25 (n¼3) 0.058 ± 0.006 (n¼3)
N338* 3832 ± 290 4.03 ± 0.80 2.54 ± 0.38 13.57 ± 1.76 (n¼3) 0.072 ± 0.038 (n¼3)
B
1
wt 625 ± 24 1.11 ± 0.12 1.6 ± 0.2 8.41 ± 0.52 (n¼7) 0.37 ± 0.06 (n¼7)
B
1
RB
2
127 ± 24 1.09 ± 0.11 ND ND ND
B
1
NB
2
511 ± 160 ND ND ND 0.28 ± 0.1 (n¼3)
B
1
CB
2
1701 ± 503 1.48 ± 0.17 1.53 ± 0.14 7.5 ± 0.6 (n¼7) 1.0 ± 0.08 (n¼3)
B
1
KB
2
1823 ± 664 ND 1.84 ± 0.25 4.1 ± 0.2
b
(n¼5) 0.7 ± 0.3 (n¼3)
B
1
KB
2
⁄SfiV 1758 ± 150 1.59 ± 0.44 1.31 ± 0.12 7.2 ± 0.8 (n¼3) 1.7 ± 0.2 (n¼3)
B
1
KB
2
⁄QGVfiKQ 2142 ± 623 ND 1.33 ± 0.12 4.6 ± 0.9
b
(n¼3) 2.0 ± 0.2 (n¼3)
B
1
KB
2
⁄VCfiCV 1786 ± 320 ND 1.42 ± 0.06 4.3 ± 0.1
b
(n¼3) 0.8 ± 0.1 (n¼3)
B
1
YB
2
2957 ± 1041 1.85 ± 1.4 1.50 ± 0.15 8.7 ± 0.8 (n¼6) 2.2 ± 0.2 (n¼3)
B
1
V323S 846 ± 128 ND 1.44 ± 0.08 4.59 ± 0.84
b
(n¼3) 0.35 ⁄0.28
a
Estimated with 10 nM[
3
H]DAK.
b
P< 0.001 vs. B
1
wt.
Role of helix 8 and C-termini in bradykinin receptors A. Faussner et al.
132 FEBS Journal 272 (2005) 129–140 ª2004 FEBS

responded to stimulation with 1 lmDAK with a dis-
tinct increase in phosphorylation. The slowly internaliz-
ing B
1
KB
2
, in contrast, exhibited no significant
phosphorylation even when challenged with DAK.
Total IP accumulation of B
1
wt and B
1
⁄B
2
chimeras parallels their agonist-inducible
internalization
The IP release was expressed as unstimulated or DAK-
stimulated accumulation of total IPs for 30 min at
37 C compared to the IP content of control cells that
had remained at 4 C. There was a clear correlation
between the agonist-inducible internalization and the
IP accumulation it could induce when stimulated
(Fig. 5). All chimeric constructs displaying rapid agon-
ist-inducible internalization (B
1
CB
2
,B
1
YB
2
,B
1
KB
2
⁄
SfiV) showed an IP response similar to that seen for
B
1
wt (8.41 ± 0.52 fold for B
1
wt and 7.2–8.7-fold for
the chimera). In contrast, the chimera that internalized
poorly (B
1
KB
2
,B
1
KB
2
⁄QGVfiKQ, B
1
KB
2
⁄VCfiCV)
showed a significantly reduced IP signal (4.1–4.6-fold)
despite the fact that they were expressed at similar
levels to the chimeras that became rapidly internalized
(Table 1). These results suggested that V323 might
play a role in the activation of phospholipase C
through B
1
wt. Indeed, exchange of V323 for a serine
in B
1
wt (construct B
1
V323S) resulted in a clearly
reduced IP response (5.28 ± 0.91 vs. 8.41 ± 0.52 for
B
1
wt; Table 1 and Fig. 5).
Discussion
Phosphorylation of serine or threonine residues in the
C-terminus of GPCRs by second messenger kinases or
specific GRKs is a requirement for receptor sequestra-
tion [23]. However, the context in which these residues
have to appear, or the receptor specificity of their
function is not very well understood.
0 5 10 15 20 25 30
0
20
40
60
80
100
Internalization [% of total]Internalization [% of total]
N338*
TSI->AAA
B2wt
I347*
A
Time [min]
0 5 10 15 20 25 30
0
20
40
60
80
100
B1RB2
B1KB2
B1CB2
B1wt
B1NB2
B1YB2
B
Time [min]
Fig. 2. Internalization of [
3
H]agonist by wild-type bradykinin recep-
tors, truncations and chimera. HEK 293 cells expressing the wild-
type receptors B
1
wt or B
2
wt, chimera thereof, or B
2
wt truncations
or mutations were preincubated with the appropriate[
3
H] agonist:
(A) < 1.5 nM[
3
H]BK; (B) 2 nM[
3
H]DAK) for 90 min on ice. Internal-
ization was started by placing the cells in a 37 C water bath and
stopped at the indicated times. Surface-bound and internalized
agonist were determined as described in Material and methods.
Agonist internalization was expressed as percentage of total bound
agonist. Results are given as mean ± SEM of at least three inde-
pendent experiments performed in triplicate.
Fig. 3. [
3
H]DAK internalization of B
1
KB
2
derived constructs. (A) Align-
ment of the relevant sequences of the B
1
CB
2
and B
1
KB
2
-derived
chimera compared to wild-type bradykinin receptor subtypes. Resi-
dues found in B
1
wt are in capital letters; those found in B
2
wt are in
lowercase. Amino acids identical to the B
1
wt sequence are indica-
ted by dashes. The residues mutated in B
1
KB
2
are in bold. To allow
comparison the sequence of rhodopsin is also shown. (B) Internal-
ization of [
3
H]DAK was performed as described in the legend to
Fig. 2. Each time point represents the mean ± SEM of at least
three different experiments done in triplicate.
A. Faussner et al.Role of helix 8 and C-termini in bradykinin receptors
FEBS Journal 272 (2005) 129–140 ª2004 FEBS 133
















