Fatty acids increase the circulating levels of oxidative
stress factors in mice with diet-induced obesity via redox
changes of albumin
Mayumi Yamato
1
, Takeshi Shiba
1
, Masayoshi Yoshida
2
, Tomomi Ide
2
, Naoko Seri
1
,
Wataru Kudou
1
, Shintaro Kinugawa
3
and Hiroyuki Tsutsui
3
1 Department REDOX Medicinal Science, Faculty of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan
2 Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
3 Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan
Metabolic syndrome is characterized by a constella-
tion of multiple risk factors, such as dyslipidemia,
hyperglycemia, hypertension, and abdominal obesity.
Several studies have focused on the role of oxidative
stress in metabolic syndrome [1–6]. For example, oxi-
dative stress markers, such as thiobarbituric acid
reactive substances (TBARS), an index of lipid perox-
idation, 8-hydroxy-2¢-deoxyguanosine, a biomarker of
oxidative DNA damage, and oxidative modification
of low-density lipoprotein, increased in the plasma of
a rat model of metabolic syndrome [6]. Plasma con-
centrations of free fatty acids are also increased in
metabolic syndrome, and might be involved in the
pathogenesis of skeletal muscle insulin resistance [7,8].
Increased fatty acids resulted in cellular damage via
the induction of oxidative stress [9,10]. In skeletal
muscle cells, palmitate-induced mitochondrial DNA
damage and cytotoxicity were caused by the overpro-
duction of peroxynitrite [9]. Zhang et al. demonstra-
ted that fatty acids enhanced monocyte adhesion to
endothelial cells in vitro and that the process was
mediated through the increased generation of reactive
oxygen species and the enhanced expression of the
integrin CD11b [10].
Keywords
ESR; albumin; fatty acid; obesity; oxidative
stress
Correspondence
H. Tsutsui, Department of Cardiovascular
Medicine, Hokkaido University Graduate
School of Medicine, Kita-15, Nishi-7, Kita-ku,
Sapporo 060-8638, Japan
Fax: +81 11 706 7874
Tel: +81 11 706 6973
E-mail: htsutsui@med.hokudai.ac.jp
(Received 11 April 2007, revised 29 May
2007, accepted 31 May 2007)
doi:10.1111/j.1742-4658.2007.05914.x
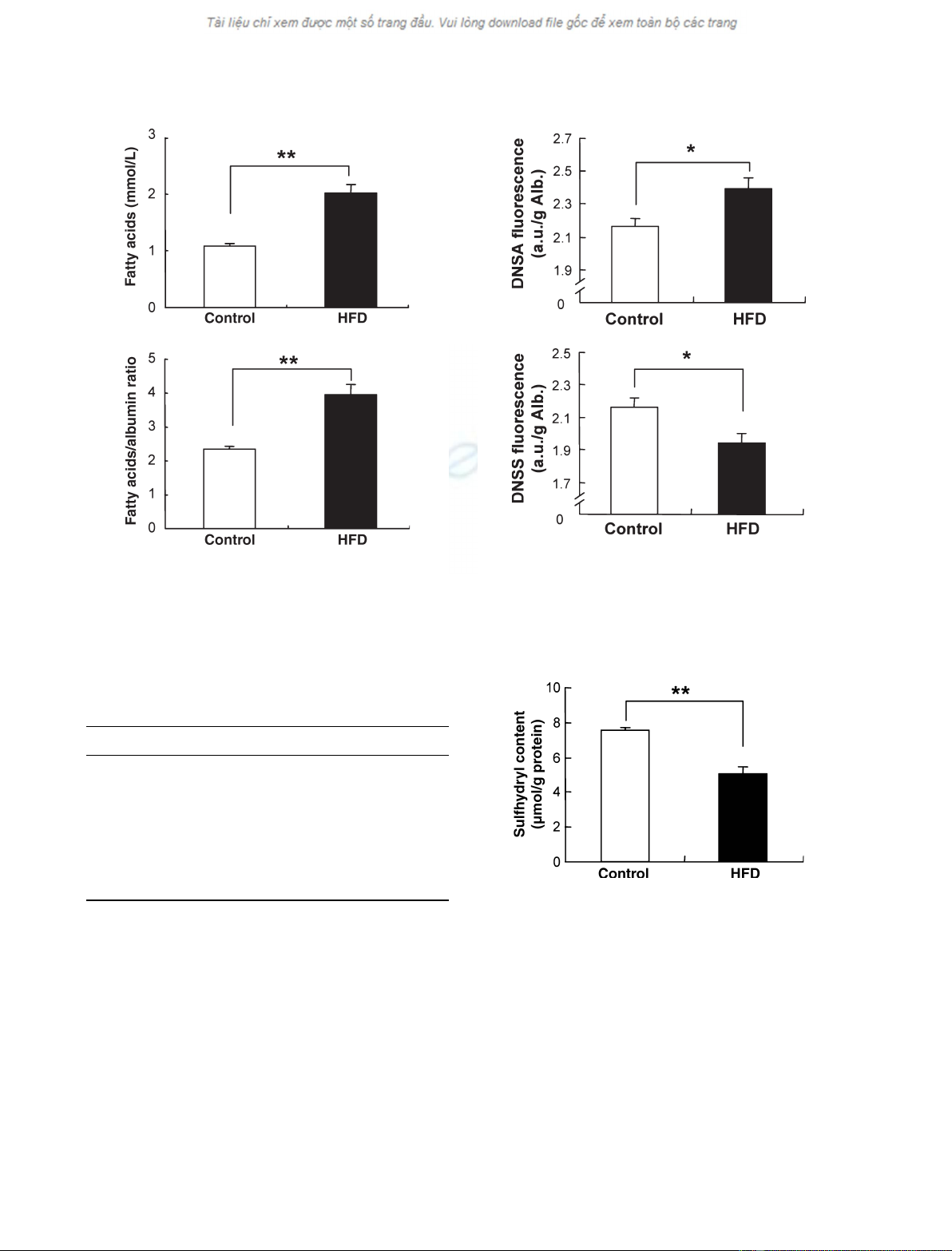
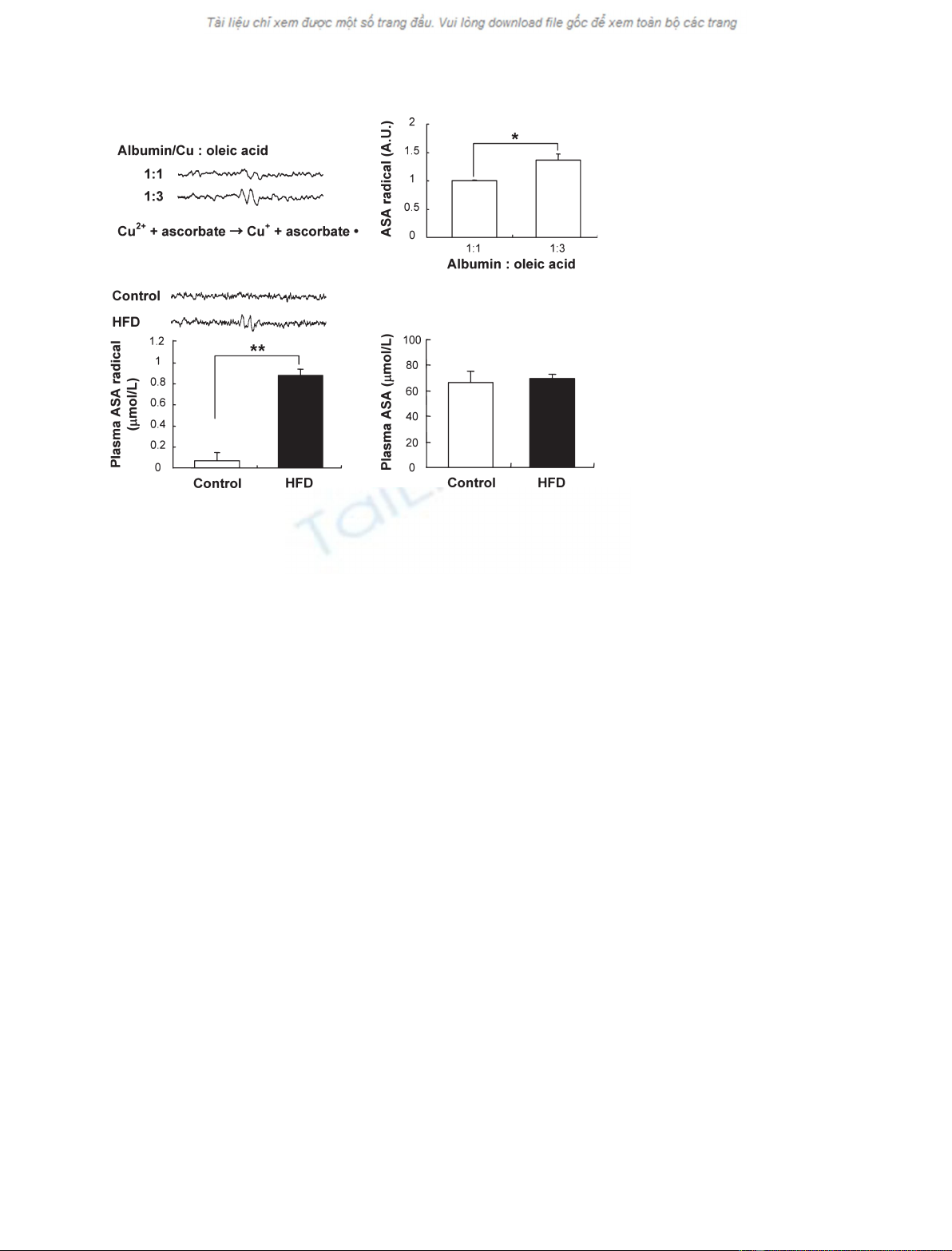
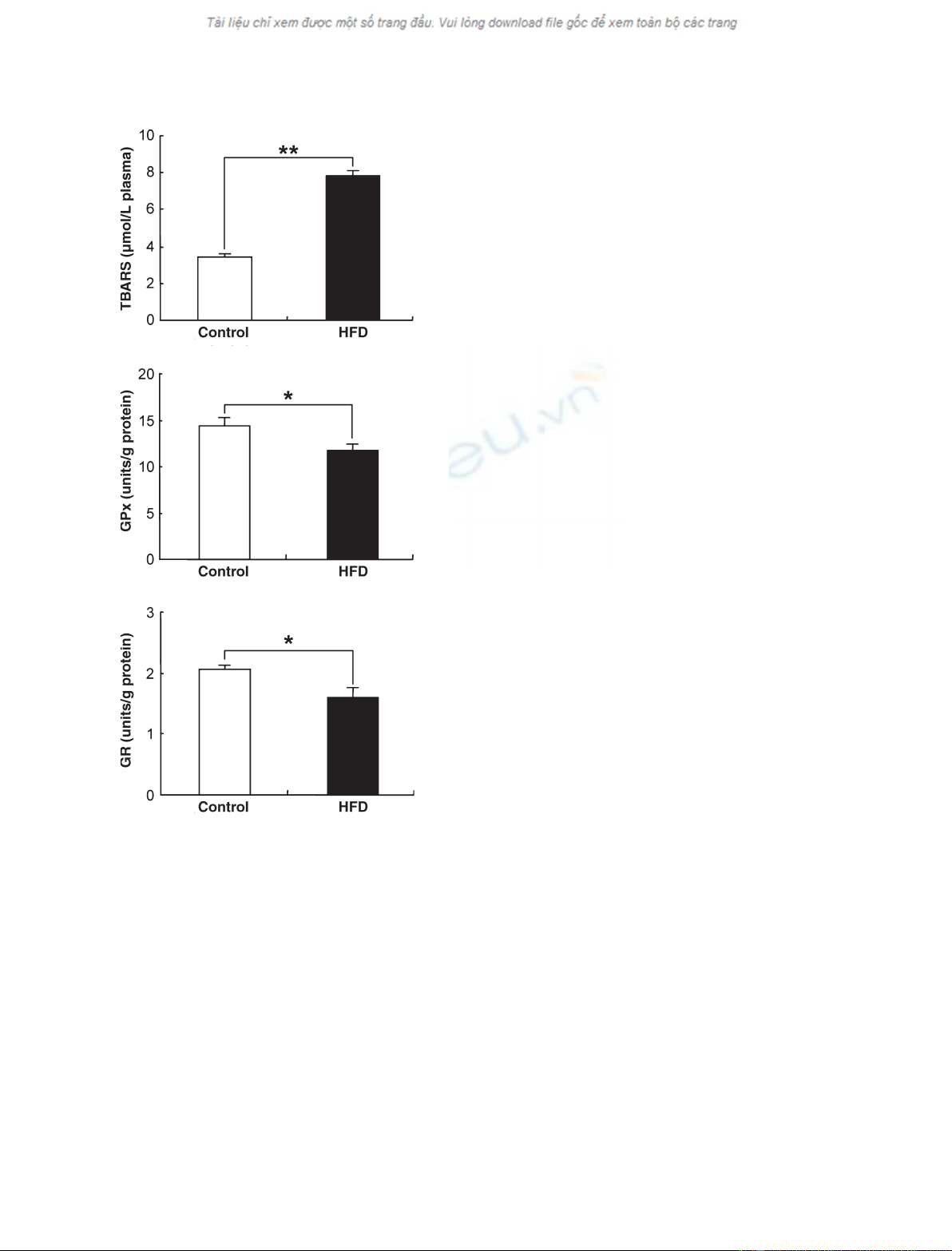
Plasma concentrations of free fatty acids are increased in metabolic syn-
drome, and the increased fatty acids may cause cellular damage via the
induction of oxidative stress. The present study was designed to determine
whether the increase in fatty acids can modify the free sulfhydryl group in
position 34 of albumin (Cys34) and enhance the redox-cycling activity of
the copper–albumin complex in high-fat diet-induced obese mice. The mice
were fed with commercial normal diet or high-fat diet and water ad libitum
for 3 months. The high-fat diet-fed mice developed obesity, hyperlipemia,
and hyperglycemia. The plasma fatty acid ⁄albumin ratio also significantly
increased in high-fat diet-fed mice. The increased fatty acid ⁄albumin ratio
was associated with conformational changes in albumin and the oxidation
of sulfhydryl groups. Moreover, an ascorbic acid radical, an index of
redox-cycling activity of the copper–albumin complex, was detected only in
the plasma from obese mice, whereas the plasma concentrations of ascorbic
acid were not altered. Plasma thiobarbituric acid reactive substances were
significantly increased in the high-fat diet group. These results indicate that
the increased plasma fatty acids in the high-fat diet group resulted in the
activated redox cycling of the copper–albumin complex and excessive lipid
peroxidation.
Abbreviations
ASA, ascorbic acid; DNSA, dansyl amide; DNSS, dansyl sarcosine; GPx, glutathione peroxidase; GR, glutathione reductase; HFD, high-fat
diet; Nbs
2
, 5,5¢-dithiobis(2-nitrobenzoic acid); TBARS, thiobarbituric acid reactive substances.
FEBS Journal 274 (2007) 3855–3863 ª2007 The Authors Journal compilation ª2007 FEBS 3855