
Journal of Water Resources & Environmental Engineering - No. 87 (12/2023)
80
Enhance removal of hospital wastewater-contaminated antibiotics by
H
2
O
2
/S
2
O
8
2-
/ZVI process
Hoa Thanh Nguyen
1*
, Nguyen Thi Phuong
2
, Nguyen Thi Lien
1
Abstract:
The study indicates efficiency of antibiotics: Amoxicillin (AMX) and ciprofloxacin (CIP)
removal in hospital wastewater using H
2
O
2
/S
2
O
82-
/ZVI process. Application of Box –
Behnken design
was to determine the experiment's effects on COD removal. Effect of pH, H
2
O
2
, S
2
O
82-
, ZVI and
reaction time as independent variables in batch oxidation experiments, namely quadratic re
sponse. The
predicted value of COD removal at optimum conditions (pH=5, [H
2
O
2
]=14.90 µg/L, [S
2
O
82-
]=50 mg/L,
ZVI=40 mg/L and reaction times: 42.92 min) were 84.97%±1.6%. The AMX and CIP removal
efficiencies were 99.95% and 99.90% within 42.92 minutes, whil
e 47.60% COD occurred within 120
minutes. As a consequence, H
2
O
2
/S
2
O
82-
/ZVI
process is an efficient and emerging method for removing
antibiotics in hospital wastewater.
Keywords: Hospital wastewater, antibiotics, advanced oxidation process, zero-valent iron.
1. Introduction
*
With the outbreak of the Covid-19 pandemic,
hospitals and medical facilities take the lives of
tens of millions, leading to the increase of
antibiotics and the generation of large amounts of
wastewater. Ciprofloxacin (CIP) and Amoxicillin
(AMX) are widely used to treat bacterial
infections of the skin, lungs or airways. Many
previous research found them in hospital influent
up 10 µg/L (Shuaa Al-Maadheed, 2019).
Antibiotics are not biodegradable and accumulate
long in ecosystems (microorganisms, plants, and
animals) and the environment. Therefore, the
study of antibiotics removal in hospital
wastewater is overly essential and factual, laying
the premise for finding the optimal environmental
method. Various techniques produce free radicals
such as OH
*
, SO
4*-
with high potential oxidation
E= 2.7V and E=2.4, respectively (Silva, 2020).
Zero-valent iron (ZVI) is frequently used to
remove numerous environmental toxins due to its
1
Faculty chemistry and environment, Thuyloi University
2,1
Mavin Group joint stock Company, Hanoi, Vietnam
*
Corresponding author
Received 29
th
Nov. 2023
Accepted 27
th
Dec. 2023
Available online 31
st
Dec. 2023
being inexpensive and ecologically safe.
Typically, ZVI can serve as a reactive catalyst by
oxidizing it to ferrous (Fe
2+
) and releasing
electrons to decrease other substances or by
reacting with strong oxidants such as hydro
peroxide or persulfate to produce strong oxidants
(hydroxyl and sulfate radicals) capable of
degrading environmental toxins, as shown in (Eqs.
(1)-(5))(Bokare and Choi, 2014)
2Fe
0
+ H
2
O + 2O
2
→2Fe
2+
+ 4OH
-
(l)
Fe
0
+ 2H
+
→ Fe
2+
+ H
2
(2)
Fe
2+
+ H
2
O
2
→ Fe
3+
+ OH
-
+ OH
*
(3)
Fe
2+
+ OH
*
→ Fe
3+
+ OH
-
(4)
Fe
3+
+ H
2
O
2
→ Fe
2+
+OH
2*
+ H
+
(5)
Fe
0
+S
2
O
82-
-> Fe
2+(aq)
+SO
42-
(6)
Fe
2+
+ S
2
O
82-
->Fe
3+
+SO
42-
+SO
4*-
(7)
Fe
3+
+ S
2
O
82-
→ Fe
2+
+ SO
42-
+SO
4*-
(8)
Fe
0(s)
+ 2Fe
3+(aq)
→3Fe
2+
(9)
S
2
O
82-
+ H
+
→ HS
2
O
8-
(10)
HS
2
O
8-
→ SO
4*-
+ SO
42-
+ H
+
(11)
SO
4*-
+ OH
-
→ SO
42-
+ OH
*
(12)
In addition, SO
4*
retains its robust and potent
oxidizing properties throughout a broad pH
range. Many research has proven that SO
4*-
is
the primary radical at pH 7; SO
4*-
and OH
*
are
performed simultaneously at neutral pH; and

Journal of Water Resources & Environmental Engineering - No. 87 (12/2023)
81
OH
*
is the predominant radical at a more basic
pH (pH >9), as stated by Eq (10)-Eq (12)
(Venkateshaiah et al., 2021). Nonetheless,
several disadvantages of SO
4*-
must still be
demonstrated. After being reduced, this radical
will become SO
42-
, which is limited below a
concentration of 250 mg/L in WHO, EC, and
Vietnam's drinking water regulations (QCVN
01-1:2018/BYT) to prevent unpleasant taste,
loose stools, and disintegration issues (Dietrich
and Burlingame, 2015). Additionally, SO
4*-
reacts with chemicals generally more slowly
than OH
*
, and OH
*
demonstrates greater
generality than SO
4*-
when removing various
contaminants from natural wastewater.
This research aim is to estimate the
integrated AOPs assisted ZVI via persulfate and
hydroperoxide for COD of hospital wastewater
treatment. Thus, the objectives of this research
are: (1) optimizing the effective parameters on
the performance of the H
2
O
2
/S
2
O
82-
/ZVI
process, and (2) determining the efficiency of
removing AMX and CIP contaminated in
hospital wastewater by H
2
O
2
/S
2
O
82-
/ZVI. These
findings would help develop and introduce an
alternative oxidation process to remove the
antibiotics from hospital wastewater.
2. Methodology
2.1. Chemicals and materials
Ciprofloxacin hydrochloride hydrate (98%),
Amoxicillin (98%), zero valent iron, Na
2
S
2
O
8
were provided by Acros Organic (Belgium).
H
2
O
2
40%, H
2
SO
4
98%, K
2
Cr
2
O
7
were acquired
from Duc Giang, Vietnam, to analyze COD. For
the water pH adjustment experiment,
hydrochloric acid (HCl 0.1 M; Xichlong, China)
and sodium hydroxide (NaOH 0.1 M; Xichlong,
China) were used.
2.2. Optimization using Box-Behken design
The experiments were designed by Design
Expert Software (version 12), based on Box-
Behnken design (BBD), and the total
experiments were 29 runs (Table 1).
BBD was used to analyze four factors:
mZVI, [H
2
O
2
], [PS] and reaction time, and each
component is conducted at three levels (-1, 1,
+1) based on previous research experience as
shown in Table 1. in which five experiments
repeated at the center with the objective
function of COD treatment efficiency. Each
experiment was repeated three times and
averaged.
Data processing: Processing experimental
data with DX12 statistical software to analyze
regression coefficients, and response surfaces
and optimize with the formal:
2
00 0 0 0
k k k k
i i ii i i j i j
i i i j
Y b b X b X bb X X
Table 1. Experimental matrix table of BBD design
Variable (X)
Factor Symbol -1 0 1
ZVI, mg/L A 40 70 100
H
2
O
2
, µg/L B 10 20 30
S
2
O
82-
, mg/L C 50 100 150
Time, min D 30 45 60
%COD, % Y
2.3. Analysis
CIP and AMX were analyzed using an
Agilent 1200 series high-performance liquid
chromatography (HPLC) system (Capell Pak
Journal of Water Resources & Environmental Engineering - No. 87 (12/2023)
82
C18 column; 250 mm 4.6 mm inner diameter)
for chromatographic separations at room
temperature. The mobile phase, namely
acetonitrile/acetic acid 0.5% mixed solution
(30:70, v/v), was vacuum filtered with a Water
Associates (Milford, MA, USA) filtering kit (w
= 0.45 µm). The flow rate of the mobile phase
was 0.5 mL min
–1
and the detection wavelength
was set at 280 nm for CIP and 23 nm for AMO.
COD was determined by Methods: 5220 C
(Closed Reflux Titrimetric Method)
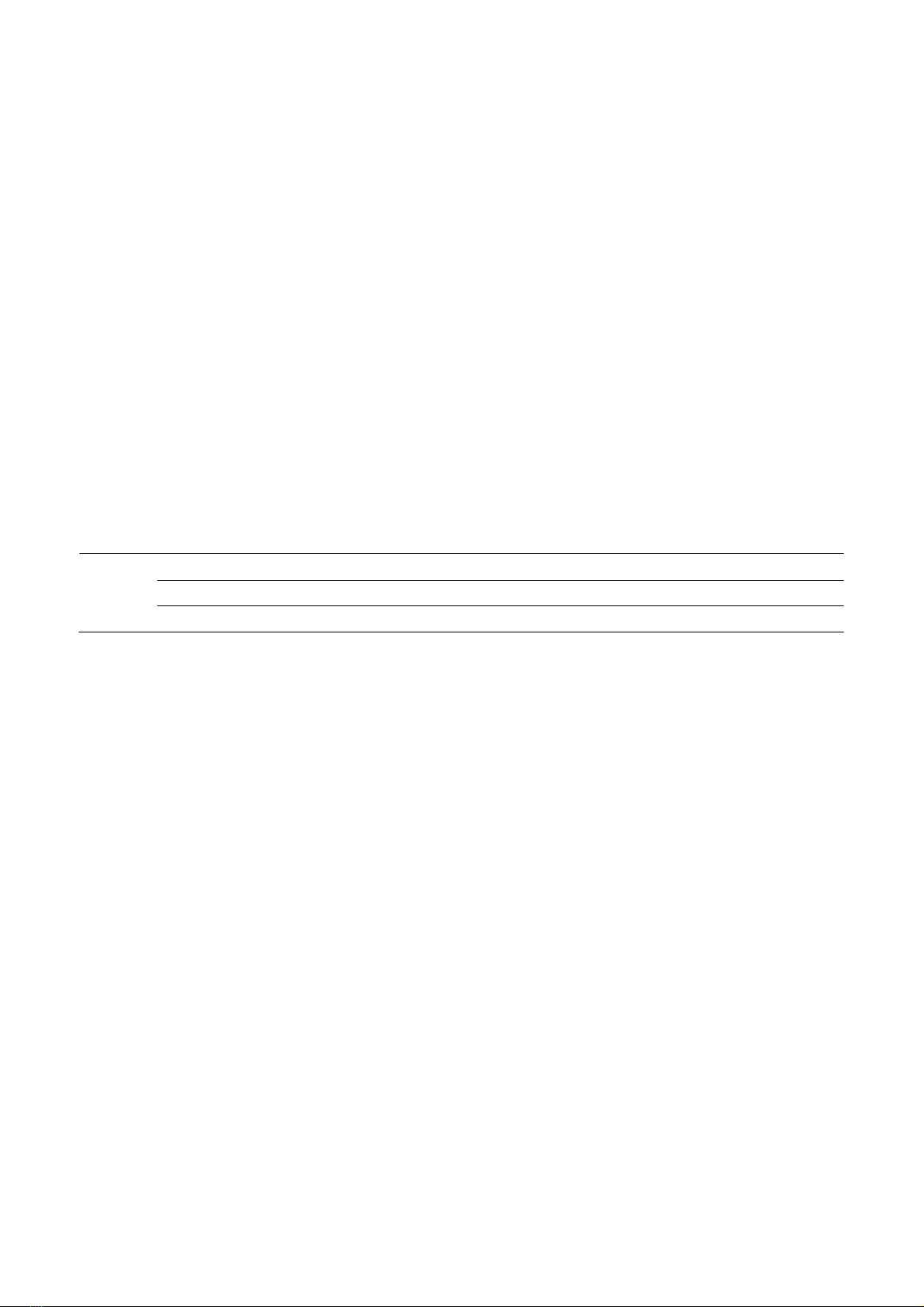
3. Results and discussion
3.1. Optimization of factors affecting COD
removal efficiency in hospital wastewater by
H
2
O
2
/S
2
O
82-
/ZVI system
The four factors experiments were conducted
according to BBD and experimental planning
using DX12 software for results (Table 2). For
the condition at the center point do as many as
five runs, namely at the time of 45 min,
[[H
2
O
2
]=20 µg/L, [PS]=100 mg/L, mZVI=70
mg/L. The percentage of COD removal from
the research results is 52.40; 52.40; 50.00;
50.00, and 50.00%, proving the results reliable.
ANOVA results in the interaction between
process variables and response variables. From
the results of ANOVA analysis (Table 3), the
model has statistical significance with a
confidence p<0.0001 for the COD removal
efficiency of 84.97%, and most of the
dependent variables explained by data.
Therefore, it proves that %COD obtained from
the model is compatible with the experiment
(Mariam, 2020).
Table 2. Experiment result data on COD removal efficiency
Value of variable Response
A: ZVI B: H
2
O
2
C: S
2
O
82-
E: Time %COD
Run
mg/L µg/L mg/L min %
1 70 20 100 45 52.40
2 100 20 50 45 42.86
3 40 20 150 45 33.33
4 70 30 100 60 40.00
5 40 20 50 45 100
6 40 20 100 60 33.33
7 70 30 50 45 90.91
8 70 20 150 30 54.55
9 70 20 50 30 54.55
10 70 20 100 45 52.40
11 70 10 100 30 44.44
12 70 20 100 45 50.00
13 100 30 100 45 44.44
14 70 20 100 45 50.00
15 40 20 100 30 44.44
16 40 30 100 45 75.00
17 70 10 150 45 80.00
18 40 10 100 45 33.33
19 100 20 100 60 33.33
20 70 30 150 45 50.00
21 100 10 100 45 72.73

Journal of Water Resources & Environmental Engineering - No. 87 (12/2023)
83
Value of variable Response
A: ZVI B: H
2
O
2
C: S
2
O
82-
E: Time %COD
Run
mg/L µg/L mg/L min %
22 70 20 50 60 57.14
23 100 20 150 45 100
24 70 20 150 60 42.86
25 70 10 50 45 54.55
26 100 20 100 30 44.44
27 70 10 100 60 20.00
28 70 20 100 45 50.00
29 70 30 100 30 36.36
Table 3. ANOVA analysis results of hospital wastewater effluent treatment to %COD removal
Source Sum of Square df Mean square F-value p-value
Prob>F
Model 11019.64 14 787.12 137.15 < 0.0001 significant
A: ZVI 28.12 1 28.12 4.90 0.0440
B: H
2
O
2
83.53 1 83.53 14.55 0.0019
C: S
2
O
82-
128.60 1 128.60 22.41 0.0003
D: Time 226.44 1 226.44 39.46 < 0.0001
AB 1223.60 1 1223.60 213.21 < 0.0001
AC 3831.23 1 3831.23 667.76 < 0.0001
AD 0 1 0 0 1.0000
BC 1100.91 1 1100.91 191.83 < 0.0001
BD 197.12 1 197.12 34.35 < 0.0001
CD 51.07 1 51.07 8.90 0.0099
A² 67.79 1 67.79 11.81 0.0040
B² 10.95 1 10.95 1.91 0.1889
C² 1696.42 1 1696.42 295.60 < 0.0001
D² 1607.39 1 1607.39 280.08 < 0.0001
Residual 80.35 14 5.74
Lack of fit 73.43 10 7.34 4.25 0.0879 not significant
Pure error 6.91 4 1.73
Cor total 11099.99 28
R
2
0.9928 Predicted R
2
0.9609
Y (%COD
predicted
) = 50,96 + 1,53A + 2,64B –
3,27C – 4,34D – 17,49 AB + 30,95AC –
16,59BC + 7,02BD – 3,57CD + 3,23A
2
+ 1,3B
2
+ 16,17C
2
– 15,74D
2
S
2
O
82-
concentration and reaction time
exhibited negative influences, while ZVI and
H
2
O
2
concentration positively affected COD
removal rate.
Journal of Water Resources & Environmental Engineering - No. 87 (12/2023)
84
Figure 1. Response surface of each pair of factors affecting COD
removal efficiency by H
2
O
2
/ S
2
O
82-
/ZVI
a) mZVI [H
2
O
2
] b) [H
2
O
2
] – [S
2
O
82-
]
c) [S
2
O
82-
] – mZVI d) mZVI – Time
From Figure 1, it appears that the four
independent variables (mZVI, [H
2
O
2
], [S
2
O
82-
]
and time) significantly influence the %COD
removal. COD is the amount of oxygen needed
to oxidize all organic matter using a strong
oxidizer chemically.
Based on the experimental data obtained,
with the help of DX12 software, it is possible to
find the optimal conditions (%COD: highest;
mZVI, [H
2
O
2
], [S
2
O
82-
] and time: least) to
conduct advanced oxidation process, COD
removal in hospital wastewater at laboratory
scale: mZVI = 40 mg/L, [H
2
O
2
]= 14.9 µg/L,
[S
2
O
82-
] = 50 mg/L and time = 42.92 min (≈43
min) with %COD
predicted
= 84.97%.
Repeat 3 experiments with the factors found
above, the results are:
%COD
retry
= 84.97 %± 1.6 %
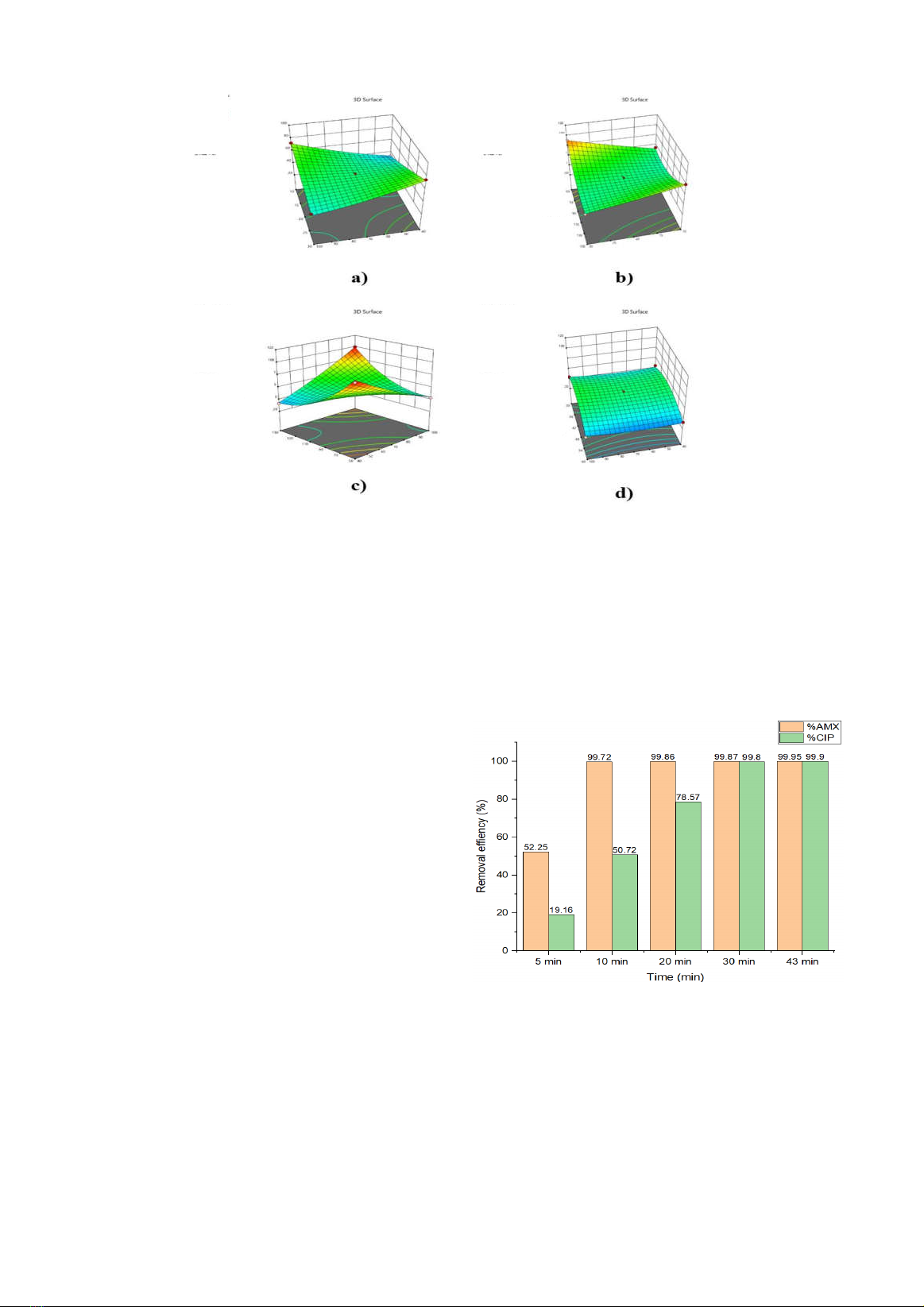
3.2. Analysis results of concentrations AMX
and CIP
To determine the efficiency of AMX and CIP
treatment in the H
2
O
2
/ S
2
O
82-
/ZVI process was
used the HPLC method analyzed the change of
AMX and CIP before and after the reaction.
Figure 2. CIP and AMX degradation by CIP
và AMX by H
2
O
2
/ S
2
O
82-
/ZVI process
Figure 2 shows that treatment efficiency
increases with response time. For CIP in the first
10 min, the efficiency is relatively slow (50.72%),
while was eliminated the performance of AMX by
A. mZVI (mg/L)
COD (%)
COD (%)
COD (%)
COD (%)
A. mZVI (mg/L)
A. mZVI (mg/L)
C. PS (mg/L)
C. PS (mg/L)
B. H
2
O
2
(mg/L)
B. H
2
O
2
(mg/L)
D. Thời gian (phút)






![Hiệu quả tiêu năng của mũi phun hai tầng [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250509/bachlapkim01/135x160/7891746785340.jpg)





![Đề thi Con người và môi trường cuối kì 2 năm 2019-2020 có đáp án [kèm file tải]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250523/oursky06/135x160/4691768897904.jpg)




![Đề cương ôn tập Giáo dục môi trường cho học sinh tiểu học [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251212/tambang1205/135x160/621768815662.jpg)








