
MINIREVIEW
Gonadotropin-releasing hormone: GnRH receptor signaling
in extrapituitary tissues
Lydia W. T. Cheung and Alice S. T. Wong
School of Biological Sciences, University of Hong Kong, China
Introduction
The hypothalamic gonadotropin-releasing hormone
(GnRH) is a decapeptide that plays a critical role in
the regulation of reproduction. GnRH-I (pGlu-His-
Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH
2
) is the first
GnRH isoform discovered in mammalian brain. Its
major role is to stimulate pituitary secretion of
gonadotropins, luteinizing hormone and follicle-stimu-
lating hormone, which in turn stimulate the gonads
for steroid production. Subsequently, a second iso-
form of GnRH (His5, Trp7, Tyr8) (GnRH-II) has
been isolated from chicken brain. It is also highly
conserved among vertebrates, including mammals [1].
However, in contrast to GnRH-I, GnRH-II is
expressed at significantly higher levels outside the
Keywords
cross-talk; extrapituitary; GnRH; GnRH
receptor; MAPK; metastasis; pituitary;
receptor tyrosine kinase; signaling; tumor
Correspondence
A. S. T. Wong, School of Biological
Sciences, University of Hong Kong, 4S-14
Kadoorie Biological Sciences Building,
Pokfulam Road, Hong Kong, China
Fax: +852 2559 9114
Tel: +852 2299 0865
E-mail: awong1@hku.hk
(Received 14 April 2008, revised 28 May
2008, accepted 11 June 2008)
doi:10.1111/j.1742-4658.2008.06677.x
Gonadotropin-releasing hormone (GnRH) has historically been known as
a pituitary hormone; however, in the past few years, interest has been
raised in locally produced, extrapituitary GnRH. GnRH receptor
(GnRHR) was found to be expressed in normal human reproductive tissues
(e.g. breast, endometrium, ovary, and prostate) and tumors derived from
these tissues. Numerous studies have provided evidence for a role of GnRH
in cell proliferation. More recently, we and others have reported a novel
role for GnRH in other aspects of tumor progression, such as metastasis
and angiogenesis. The multiple actions of GnRH could be linked to the
divergence of signaling pathways that are activated by GnRHR. Recent
observations also demonstrate cross-talk between GnRHR and growth fac-
tor receptors. Intriguingly, the classical G
aq
–11-phospholipase C signal
transduction pathway, known to function in pituitary gonadotropes, is not
involved in GnRH actions at nonpituitary targets. Herein, we review the
key findings on the role of GnRH in the control of tumor growth, progres-
sion, and dissemination. The emerging role of GnRHR in actin cytoskele-
ton remodeling (small Rho GTPases), expression and ⁄or activity of
adhesion molecules (integrins), proteolytic enzymes (matrix metalloprotein-
ases) and angiogenic factors is explored. The signal transduction mecha-
nisms of GnRHR in mediating these activities is described. Finally, we
discuss how a common GnRHR may mediate different, even opposite,
responses to GnRH in the same tissue ⁄cell type and whether an additional
receptor(s) for GnRH exists.
Abbreviations
EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK, extracellular signal-related kinase; FAK, focal adhesion
kinase; FGF, fibroblast growth factor; GnRH, gonadotropin-releasing hormone; GnRHR, gonadotropin-releasing hormone receptor;
JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-jB, nuclear factor kappa B; PI3K,
phosphatidylinositol 3-kinase; PKC, protein kinase C; Pyk2, proline-rich tyrosine kinase 2; RTK, receptor tyrosine kinase; uPA, urokinase-type
plasminogen activator; VEGF, vascular endothelial growth factor.
FEBS Journal 275 (2008) 5479–5495 ª2008 The Authors Journal compilation ª2008 FEBS 5479

brain and is particularly abundant in the kidney,
bone marrow, and prostate [2]. This leads to the
speculation that GnRH-II may have distinct physio-
logical functions from those of GnRH-I. In line with
this is the observation that although GnRH-II can
stimulate gonadotropin secretion, its efficiency is
much lower than that of GnRH-I (only about 2% of
that of GnRH-I) [3]. This suggests that the primary
role of GnRH-II is not in the regulation of gonado-
tropin secretion. Instead, this peptide has been shown
to act as a neuromodulator [4]. The exact actions of
GnRH-II in peripheral tissues are not entirely under-
stood, but this is certainly an important topic for
investigation which may offer an opportunity to eluci-
date the undisclosed complexity of GnRH.
In this minireview, we will focus on recent progress
in understanding the roles of GnRH-I and GnRH-II
in extrapituitary tissues, in particular its emerging
role in tumor growth, invasion, and metastasis. We
will also describe the molecular mechanisms underlying
these effects, focusing on the roles of proteolysis,
adhesion, and signaling, as well as our still-emerging
understanding of receptor cross-talk with other
pathways. Finally, we will discuss two important
outstanding questions in the field regarding what might
distinguish the different responses to the same ligand
(GnRH) and whether an additional receptor(s) for
GnRH exists in humans.
Localization of GnRH receptor (GnRHR)
in peripheral reproductive tissues
The initial interest in extrapituitary GnRHR stemmed
primarily from observations in the 1980s that GnRH
analogs can inhibit the growth of nonpituitary tumor
cell lines [5]. Soon after this, a functional type I
GnRHR was demonstrated in a variety of normal
human reproductive tissues (e.g. breast, endometrium,
ovary, and prostate) and tumors derived from these
tissues.
In the ovary, GnRHR mRNAs are expressed in
granulosa-luteal cells, and increased expression of
GnRHR correlates with follicular growth and develop-
ment [6]. GnRHR binding has been demonstrated in
luteinized granulosa cells, late follicles and developing
corpora lutea, but not in primordial, early antral and
preovulatory follicles [7,8]. This stage-specific expres-
sion of GnRHR in the human granulosa and luteal
cells suggests a role for GnRH in the regulation of
ovarian physiology, particularly ovulation, follicular
atresia and luteolysis. The presence of GnRHR protein
and mRNA has also been demonstrated in human
ovarian tumor specimens, ovarian cancer cell lines and
their tissue of origin, ovarian surface epithelium [9,10].
Interestingly, levels of GnRHR seem to be associated
with cancer grading and have been reported to be
elevated in advanced stage (stages III and IV) as
compared to early stage (stages I and II) ovarian
carcinomas [11]. Our recent findings that GnRH can
promote the motility and invasiveness of ovarian can-
cer cells further corroborate the view that GnRH may
play a crucial role in tumor progression ⁄metastasis
[12,13], and these findings will be discussed in a later
section.
Using [
125
I][d-Trp6]GnRH, specific receptor binding
has been detected in membranes from 24 of 31 (77%)
endometrial carcinomas and from three of 13 (23.1%)
nonmalignant human endometrial specimens [14].
GnRHR mRNA has been clearly detected in surgical
endometrial carcinoma specimens and endometrial
carcinoma cell lines [15,16]. As with normal myome-
trium, most benign neoplasms studied thus far,
including uterine leiomyoma, also possess GnRHR
[17].
Early studies showed that the human placenta con-
tains specific binding sites for GnRH that interact with
GnRH agonists and antagonists [18]. Later on,
GnRHR was localized to the cytotrophoblast and
syncytiotrophoblast cell layers [19,20]. Temporal
expression of GnRHR in the placental cells at different
weeks of gestation has been observed, in parallel with
the time-course of chorionic gonadotropin secretion
during pregnancy [21], suggesting that the expression
of the receptor is a function of pregnancy stage.
The presence of GnRHR has been demonstrated in
numerous human breast cancer cell lines and tumor
biopsy specimens [22–24]. GnRHR was immunolocal-
ized in the cytoplasm in 37 of 58 (64%) invasive ductal
carcinoma cases [23]. The expression of GnRHR in
normal human breast tissue is still controversial, but
the sample size may have been too small to allow any
definite conclusion [22,25].
GnRHR is also present in prostate cancer cells, as
shown by radioligand-binding studies, PCR, and
western blotting analysis [26,27]. GnRHR immunore-
activity is localized to the luminal and basal epithelial
cells in benign and malignant prostate tissues. In this
study, the relative GnRHR mRNA levels showed a wide
range of individual differences that were unrelated to
the histological grades of the 16 cases [27]. There does,
however, appear to be significantly higher expression of
GnRHR in hormone-refractory prostate carcinoma
than in other types of prostate tumor (n= 80) [28].
Although these extrapituitary GnRHRs share the
same cDNA nucleotide sequence and encode tran-
scripts and proteins of the same size as the pituitary
GnRH receptor signaling L. W. T. Cheung and A. S. T. Wong
5480 FEBS Journal 275 (2008) 5479–5495 ª2008 The Authors Journal compilation ª2008 FEBS

GnRHR [20,26,29], they also differ in several ways.
First, cell surface receptor expression in extrapituitary
sites is low as compared to that of the pituitary
[15,27]. This may underlie the greater effect of the
GnRHR ligands on the gonadotropes. Second, there
are at least two classes of GnRHR: one has high affin-
ity [with nanomolar dissociation constants (K
d
)] for
GnRH, and one has low affinity (with micromolar K
d
values) for GnRH. The high-affinity GnRH-binding
sites are commonly regarded as being the same as the
GnRHR of the pituitary gland. Whereas in most of
the reported cases, both the low-affinity and high-affin-
ity GnRHR have been found in extrapituitary tissues
[30–33], in some cases, only low-affinity GnRHR could
be detected [10,18,34], and in others, e.g. in endome-
trial cancers and nonmalignant endometrial specimens,
only the high-affinity GnRHR has been demonstrated
[14]. The exact role of each of these receptors and the
implications of differential levels of expression remain
to be elucidated.
Functions of GnRH-I and GnRH-II in
cancers
Tumor growth
Over the last two decades, both GnRH agonists and
antagonists have been widely used as therapeutics in
treating sex steroid-dependent tumors. The majority
of these GnRH analogs, when given continuously,
inhibit gonadotropin synthesis and secretion via
downregulation of the pituitary GnRHRs. This indi-
rect mechanism of action has provided the rationale
for the use of GnRH analogs in the treatment of hor-
mone-dependent tumors for many years. Only since
the detection of GnRHR in extrapituitary tissues has
there been increasing interest in its direct action on
tumor cells.
GnRH-I analogs have direct antiproliferative effects
on ovarian cancer cells, which is linked to the disrup-
tion of the cell cycle at G
0
⁄G
1
[31,35,36]. On the other
hand, several independent in vitro studies failed to
demonstrate significant growth inhibition by GnRH-I
agonists, even at fairly high concentrations (micromolar
range) [37,38]. In fact, a biphasic impact of GnRH-I
agonists on growth has been reported: whereas GnRH-I
agonists at high dose (1 lm) inhibit cell proliferation
in vitro, cells treated with agonists at low dose (10 nm)
show significant growth stimulation [39]. Further
studies demonstrated that nanomolar concentrations of
GnRH-I agonists also increase cell survival under
multiple stress conditions, including DNA replication-
specific cytotoxic agents and UV radiation [40].
GnRH-II has antiproliferative effects on ovarian cancer
cells [41–43]. Although it has been suggested that this
effect of GnRH-II is mediated through the type I
GnRHR [43], there are other findings implicating a
type I GnRHR-independent action [41,42].
It is interesting to note that although both GnRH-I
agonists and antagonists exert antiproliferative effects,
the effects of GnRH-I antagonists are stronger than
those of the agonists [44]. This difference has also been
seen in an in vivo model, which demonstrates a signifi-
cant inhibition of tumor growth by GnRH-I antago-
nists but not GnRH-I agonists [45]. The advantage of
GnRH antagonists over the agonistic peptides is prob-
ably due to the fact that they inhibit the secretion of
gonadotropins and reduce sex steroid levels immedi-
ately after application, thus achieving rapid therapeutic
effects, whereas repeated exposure to agonistic agents
is required to induce functional desensitization of the
gonadotropes [46].
Treatment of human endometrial cancer cells (cell
line Ishikawa) with the GnRH-I antagonist SB-75
results in growth inhibition, mainly due to the Fas ⁄Fas
ligand-mediated apoptotic pathway, whereas GnRH-I
agonists have no effect on the same cell line [15,47,48].
Another endometrial carcinoma cell line, HEC-1A, also
exhibits differential responses to different GnRH agon-
ists and antagonists [15,30,36,48]. GnRH-II has been
shown to have antiproliferative effects on endometrial
carcinoma cells [41]. The effects of GnRH-I are
abrogated after type I GnRHR knockout [36], whereas
those of the GnRH-I antagonist cetrorelix and of
GnRH-II persist [41]. These findings suggest that the
antiproliferative effects of cetrorelix and GnRH-II are
not mediated through the type I GnRHR.
GnRH-I has been demonstrated to have antiprolifer-
ative effects on prostate cancer cells [49–51], except in
one in vivo study [52]. This antiproliferative effect
appears to be independent of the androgen receptor
status of the prostate carcinoma cells, as both andro-
gen-sensitive LNCaP cells and androgen-resistant
DU-145 cells remain sensitive to GnRH [49,50]. Acti-
vation of GnRHR may mediate these effects via direct
induction of apoptosis through caspase activation [53].
Compatible with a role for GnRH in survival at low
doses, an enhancing effect of GnRH was observed
when cells were treated with a low concentration
(100 pm) of GnRH-I agonist [54]. GnRH-II was shown
to have an antiproliferative effect on DU-145 cells and
growth-stimulatory effect on TSU-Pr1 cells, but the
type I GnRHR was not involved [55].
The influence of GnRH on the growth of human
breast cancer cells was first studied with MCF-7 cells
[56], and both in vitro and in vivo proliferation of
L. W. T. Cheung and A. S. T. Wong GnRH receptor signaling
FEBS Journal 275 (2008) 5479–5495 ª2008 The Authors Journal compilation ª2008 FEBS 5481

breast cancer cells could be inhibited by both agonistic
and antagonistic analogs of GnRH [57,58]. However,
higher efficiency of GnRH antagonists in growth inhi-
bition than that of GnRH agonists has been reported
[24,58].
Invasion and metastasis
The observation that GnRH controls tumor growth
suggests a regulatory role for this peptide in the meta-
static behavior of cancer cells. This hypothesis is sup-
ported by studies showing that GnRH-I and GnRH-II
can affect the expression of several extracellular
matrix-degrading enzymes in human extravillous cyto-
trophoblasts and decidual stromal cells to facilitate
implantation [59,60]. However, its potential role in
cancer metastasis has just begun to be revealed.
Metastasis is a complex phenomenon that requires
several specific steps, such as decreased adhesion,
increased motility, and proteolysis. The effects of GnRH
in tumor metastasis are mediated through the regulation
of adhesion molecules, Rho GTPases, and two families
of metastasis-related proteinases, the matrix metallopro-
teinases (MMPs) and the urokinase-type plasminogen
activator (uPA) system, at several levels: mRNA
transcription, secretion, and proenzyme activation.
The ability of GnRH to regulate metastasis was first
reported in melanoma cells [61]. High doses of GnRH-I
analog, at micromolar concentrations, significantly
reduces the ability of melanoma cells to invade
and migrate [61]. Preliminary data (R. M. Moretti,
M. Monagnani Marelli, J. C. van Groeninghen, M.
Motta & P. Limonta, unpublished results, 2003) indicate
that this inhibitory action is due to the effects of
integrins and MMPs [62].
We were the first to report possible metastatic activ-
ity of GnRH-I in tumors of the female reproductive
tract [12]. GnRH-I exerts a biphasic effect on cellular
migration and invasion: whereas lower (nanomolar)
concentrations of the GnRH-I agonist stimulate cellu-
lar migration and invasion in a dose-dependent man-
ner, high (micromolar) concentrations are not as
efficient. This proinvasive effect is mediated through
activation of metastasis-related proteinases, in particu-
lar MMP-2 and MMP-9 [12]. Moreover, GnRH-I is
able to transactivate the MMP-2 and MMP-9 promot-
ers, which means that GnRH can be considered to be
a new member of MMP-2 and MMP-9 transcriptional
modulators. Like GnRH-I, native GnRH-II and its
synthetic analog also induce a similar biphasic regula-
tion of ovarian cancer invasion [13]. The finding that
small interfering RNA-mediated downregulation of
type I GnRHR completely reversed the effects of both
GnRH-I and GnRH-II on cell invasion supports the
view that the same receptor, type I GnRHR, is essen-
tial for the effects of GnRH-I and GnRH-II in ovarian
cancer cells.
The decrease in uPA activity of cytosol from Dun-
ning R3327H rat prostate tumors after treatment with
GnRH-I analogs suggests that GnRH may be an
important factor in reducing the invasiveness of pros-
tate cancer [63]. High doses of GnRH-I agonists and
antagonists have been reported to attenuate the
invading capacity of both androgen-dependent and
androgen-independent prostate cancer cells by modu-
lating E-cadherin-mediated cell–cell contacts and pro-
duction of uPA and its inhibitor (plasminogen
activator inhibitor-1) [64–66]. GnRH has also been
shown to regulate cell motility through its interaction
with the small GTPases Rac1, Cdc42, and RhoA,
which are involved in the regulation of actin polymer-
ization [67].
Up to now, there has been only one study, by Von
Alten et al., investigating the role of GnRH in breast
cancer metastasis, using a coculture system with
human osteosarcoma cells to analyze tumor cell
invasion to bone [68]. The consequences of GnRHR
activation are complex and appear to be cell context
dependent: whereas treatment of cells with the
GnRH-I agonist triptorelin, the GnRH-II agonist
[d-Lys6]GnRH-II and the GnRH-I antagonist cetrorelix
decreases the invasion rate in most breast cancer cell
lines, these agents have no significant effect in the
GnRHR-positive MDA-MB-435 cells [68]. Further
investigations are required to elucidate the reason why
the MDA-MB-435 cell line reacts differently.
Organ-specific homing and colonization of cancer
cells are important and interesting features of metasta-
sis. A role for GnRH has also been suggested in the
regulation of the immune response and metastasis.
GnRH-I and GnRH-II are expressed in human normal
and cancerous T-cells. GnRH triggers laminin receptor
gene expression, adhesion to laminin, in vitro chemo-
taxis, and in vivo homing to specific organs [69].
Angiogenesis
Angiogenesis is crucial to a number of physiological
and pathological processes, such as reproduction,
development, and tissue repair, as well as tumor
growth and metastasis. Vascular endothelial growth
factor (VEGF) is implicated as the most important
angiogenesis inducer, because of its potency in a
variety of normal and tumor cells. Other angiogenic
factors include fibroblast growth factor (FGF), plate-
let-derived growth factor and the angiopoietin family.
GnRH receptor signaling L. W. T. Cheung and A. S. T. Wong
5482 FEBS Journal 275 (2008) 5479–5495 ª2008 The Authors Journal compilation ª2008 FEBS
The effect of GnRH on angiogenesis in the ovary, in
which this neovascularization is necessary for follicular
and luteal function, has been demonstrated. A recent
in vivo study using rats revealed that an application of
the GnRH-I agonist leuprolide acetate decreases the
protein expression of VEGF and angiopoietin-1 and
their receptors in ovarian follicles, and that this can be
reversed by coinjection of the GnRH antagonist antide
[70]. A similar inhibitory effect on angiogenesis can be
observed in marmosets injected with the GnRH-I
antagonist antarelix [71]. However, VEGF mRNA
expression is unaffected by the treatment. The clinical
response of uterine shrinkage after GnRH analog
treatment and a pathological role of FGF-2, VEGF
and platelet-derived growth factor in uterine leiomy-
oma growth and vascularization has also been sug-
gested [72]. Considering that angiogenesis is an
important process in many human cancers, it would be
very interesting to determine whether GnRH also plays
a key role in tumor angiogenesis.
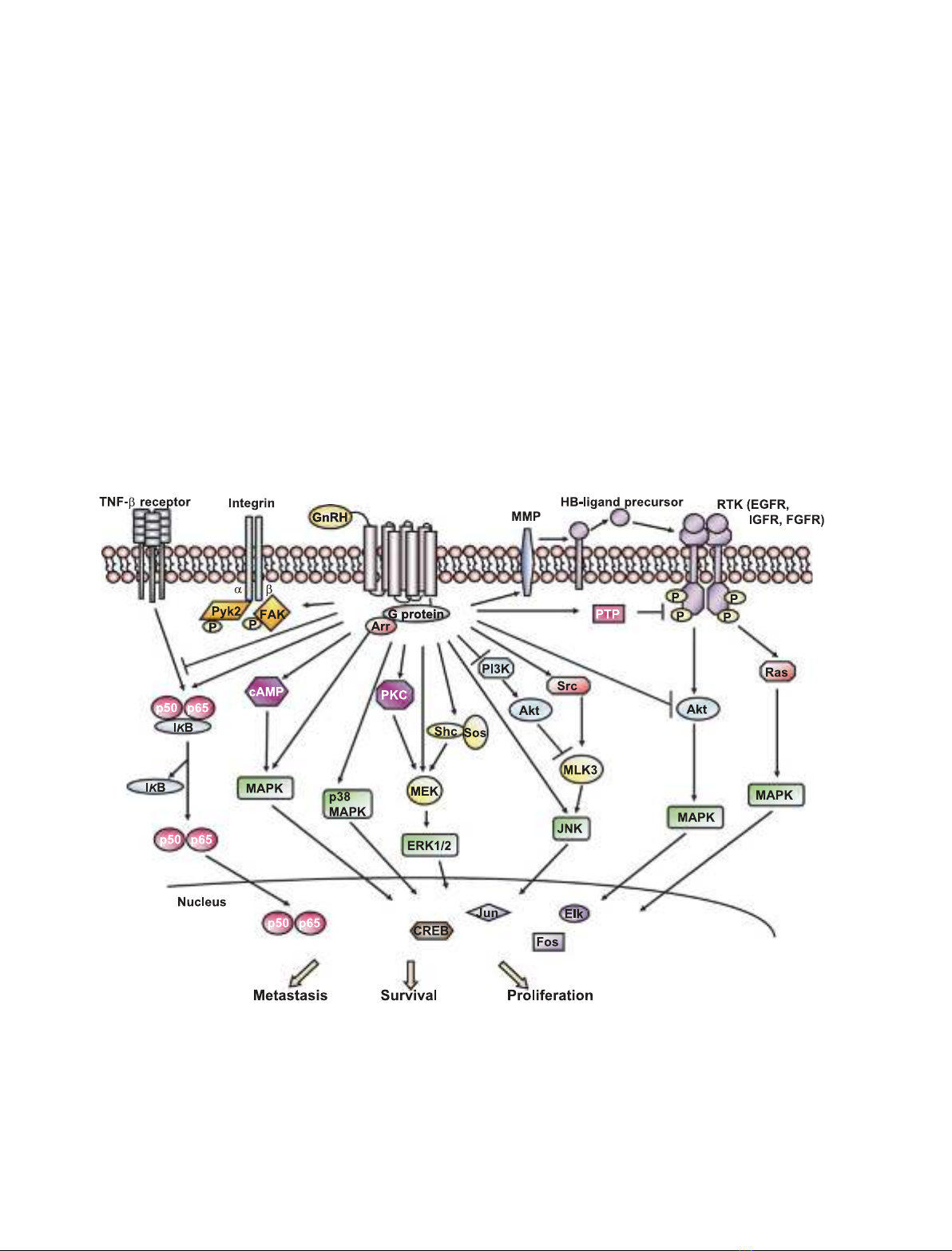
Intracellular signal transduction
Upon GnRH binding, GnRHR undergoes a conforma-
tional change and stimulates a unique G-protein. Inter-
estingly, the classical G
aq
–11-phospholipase C signal
transduction pathway, which is known to operate in
the pituitary, is not involved in the antitumor activity
of GnRH analogs. Rather, GnRHRs couple to G
ai
in
these tumors and result in the activation of several
downstream signaling cascades [73,74], such as mito-
gen-activated protein kinase (MAPK), phosphatidyl-
inositol-3-kinase (PI3K), and nuclear factor kappa B
(NF-jB) signaling. The GnRH-induced signaling path-
ways in extrapituitary tissues are shown schematically
in Fig. 1.
Fig. 1. Schematic representation of GnRHR signaling in extrapituitary tissues. Binding of GnRH to GnRHR triggers several intracellular signal-
ing cascades and cross-talk with mitogenic signaling, depending on the cell context. Some of these signaling modules can transduce extra-
cellular signals to the nucleus and thereby regulate genes that are involved in cell growth, metastasis, or survival. Arr, b-arrestin; CREB,
cAMP response element-binding protein; FGFR, fibroblast growth factor receptor; HB-EGF, heparin-binding EGF; IjB, inhibitory factor kap-
pa B; IGFR, IGF receptor; MEK, mitogen-activated protein kinase kinase; MLK3, mixed-lineage kinase 3; PTP, protein tyrosine phosphatase;
Sos, son of sevenless; TNF-a, tumor necrosis factor alpha.
L. W. T. Cheung and A. S. T. Wong GnRH receptor signaling
FEBS Journal 275 (2008) 5479–5495 ª2008 The Authors Journal compilation ª2008 FEBS 5483

![Liệu pháp nội tiết trong mãn kinh: Báo cáo [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2024/20240705/sanhobien01/135x160/4731720150416.jpg)














![Báo cáo seminar chuyên ngành Công nghệ hóa học và thực phẩm [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250711/hienkelvinzoi@gmail.com/135x160/47051752458701.jpg)









