
The ‘pair of sugar tongs’ site on the non-catalytic domain C
of barley a-amylase participates in substrate binding and
activity
Sophie Bozonnet
1,2
, Morten T. Jensen
2
, Morten M. Nielsen
1
, Nushin Aghajari
3
, Malene H. Jensen
3
,
Birte Kramhøft
1,2
, Martin Willemoe
¨s
1,2
, Samuel Tranier
3
, Richard Haser
3
and Birte Svensson
1,2
1 Enzyme and Protein Chemistry, BioCentrum-DTU, Technical University of Denmark, Kgs. Lyngby, Denmark
2 Carlsberg Laboratory, Valby, Denmark
3 Laboratoire de BioCristallographie, Institut de Biologie et Chimie des Prote
´ines, Universite
´de Lyon, France
a-amylases (EC 3.2.1.1) are endo-hydrolases acting on
a)1,4-glucosidic bonds in starch and related poly- and
oligosaccharides. They belong to the very large glyco-
side hydrolase family 13 (GH13) that, together with
GH70 and GH77, forms glycoside hydrolase clan
H (GH-H), representing about 30 enzyme specificities
(http://www.cazy.org). Secondary carbohydrate-bind-
ing sites are found either on the surface of the catalytic
structural unit or on a separate carbohydrate-binding
module (CBM) in some of the GH-H members [1].
Keywords
barley a-amylase; crystal structures;
secondary carbohydrate-binding sites;
starch granules; surface plasmon resonance
Correspondence
B. Svensson, Enzyme and Protein
Chemistry, BioCentrum-DTU, Technical
University of Denmark, Søltofts Plads, Bldg
224, DK-2800 Kgs. Lyngby, Denmark
Fax: +45 45 88 63 07
Tel: +45 45 25 27 40
E-mail: bis@biocentrum.dtu.dk
(Received 1 June 2007, revised 18 July
2007, accepted 1 August 2007)
doi:10.1111/j.1742-4658.2007.06024.x
Some starch-degrading enzymes accommodate carbohydrates at sites situ-
ated at a certain distance from the active site. In the crystal structure of
barley a-amylase 1, oligosaccharide is thus bound to the ‘sugar tongs’ site.
This site on the non-catalytic domain C in the C-terminal part of the mole-
cule contains a key residue, Tyr380, which has numerous contacts with the
oligosaccharide. The mutant enzymes Y380A and Y380M failed to bind to
b-cyclodextrin-Sepharose, a starch-mimic resin used for a-amylase affinity
purification. The K
d
for b-cyclodextrin binding to Y380A and Y380M was
1.4 mmcompared to 0.20–0.25 mmfor the wild-type, S378P and S378T
enzymes. The substitution in the S378P enzyme mimics Pro376 in the bar-
ley a-amylase 2 isozyme, which in spite of its conserved Tyr378 did not
bind oligosaccharide at the ‘sugar tongs’ in the structure. Crystal structures
of both wild-type and S378P enzymes, but not the Y380A enzyme, showed
binding of the pseudotetrasaccharide acarbose at the ‘sugar tongs’ site. The
‘sugar tongs’ site also contributed importantly to the adsorption to starch
granules, as K
d
¼0.47 mgÆmL
)1
for the wild-type enzyme increased to
5.9 mgÆmL
)1
for Y380A, which moreover catalyzed the release of soluble
oligosaccharides from starch granules with only 10% of the wild-type activ-
ity. b-cyclodextrin both inhibited binding to and suppressed activity on
starch granules for wild-type and S378P enzymes, but did not affect these
properties of Y380A, reflecting the functional role of Tyr380. In addition,
the Y380A enzyme hydrolyzed amylose with reduced multiple attack,
emphasizing that the ‘sugar tongs’ participates in multivalent binding of
polysaccharide substrates.
Abbreviations
AMY1 and AMY2, barley a-amylases 1 and 2; BASI, barley a-amylase ⁄subtilisin inhibitor; b-CD, b-cyclodextrin; CBM, carbohydrate-binding
module; CBM20, carbohydrate-binding module family 20; Cl-pNPG
7
, 2-chloro-4-nitrophenyl b-D-maltoheptaoside; cv, column volume; DMA,
degree of multiple attack; DP, degree of polymerization; GH13, glycoside hydrolase family 13; GH-H, glycoside hydrolase clan H; iBS,
insoluble blue starch; RU, response unit; SBD, starch-binding domain; SPR, surface plasmon resonance; thio-DP4, methyl-4¢,4¢¢,4¢¢¢-
trithiomaltotetraoside.
FEBS Journal 274 (2007) 5055–5067 ª2007 The Authors Journal compilation ª2007 FEBS 5055

Plant a-amylases mobilize starch in plastids, tubers
and seeds, and barley isozyme 1 and 2 (AMY1 and
AMY2) are de novo synthesized in seed aleuron layers
at germination encoded by two multigene families
of 80% sequence identity and > 95% identity within
a subfamily. Only one AMY1 and two AMY2 iso-
forms were found in germinating seeds from a total of
10 barley a-amylase encoding genes; these three pro-
teins moreover underwent differential degradation dur-
ing germination [2]. AMY1 and AMY2 have virtually
identical three-dimensional structures composed of an
N-terminal catalytic (b⁄a)
8
-barrel (domain A), a
domain B, protruding between b-strand 3 and a-helix
3, and a C-terminal antiparallel b-sheet domain-C
[3,4]. The isozymes show functional and stability dif-
ferences and roles of selected amino acid residues were
characterized by mutational analysis [5–12]. The A and
B domains together form the active site [3,4]. Domain
B is also associated with effects of Ca
2+
on stability
and activity [5,13] and with the AMY2-specific sensi-
tivity to barley a-amylase ⁄subtilisin inhibitor (BASI)
[5,14,15]. AMY1 furthermore binds substrates – starch
granules included – more tightly than does AMY2,
which shows a higher turn-over rate than AMY1
[16–18]. Domain-C is present in almost all GH-H
members and its functional role has not yet been
assigned. Remarkably, the ‘sugar tongs’ site defined
around Tyr380 in domain-C of AMY1 and binding
malto-oligosaccharide [4] was not occupied in the
structure of AMY2 [3] although this critical tyrosine is
conserved in AMY2.
AMY1, AMY2, and other GH-H enzymes possess
different secondary carbohydrate-binding sites that are
not part of the active site area but which are situated
on the surface of the catalytic domain or an inti-
mately associated domain rather than on a CBM, e.g.
a starch-binding domain (SBD) [1,3,4,19–22]. The role
of multivalent binding in enzymatic degradation of
polysaccharides is in general not clearly understood at
the molecular level. In amylolytic enzymes such sites
are thought to (a) ensure association with starch gran-
ules, (b) assist in disentangling of a-glucan chains,
(c) guide the substrate chain to the active site, and
(d) confer allosteric regulation. Multivalent binding is
also envisaged in the multiple attack mechanism
proposed in the late 1960s for amylose degradation
by a-amylase, in which an initial endo-attack was
followed by hydrolysis of more glucosidic bonds
before the enzyme–substrate complex dissociated [23].
Multiple attack was later described for cellulases,
chitinases, and pectinases and termed processivity [24].
Barley AMY1 hydrolyzes amylose with a degree of
multiple attack (DMA) of 2; thus, after the initial
cleavage, two substrate bonds were hydrolyzed with
release of shorter products [12]. Whereas DMA was
mostly reduced for AMY1 mutants in the substrate-
binding cleft, DMA values of 3.0 and 3.3 were found,
respectively, for an AMY1–SBD fusion [25] having an
SBD attached to the AMY1 C-terminus, and for the
AMY1 Y105A mutant at the high-affinity subsite )6
[12]. However, because maltoheptaose was the major
product released by wild-type AMY1 and all of the
different variants, it was suggested that amylose was
attached to the enzyme surface also outside the
substrate-binding cleft [12]. The ‘sugar tongs’ in
domain-C [4,21] seemed an obvious candidate for
such a binding site.
Tyr380 cOH moved 3.1 A
˚when the ‘sugar tongs’
captured a ligand [4,21] and the engagement of Tyr380
in eight of 17 protein contacts with methyl-4¢,4¢¢,4¢¢¢-
trithiomaltotetraoside (thio-DP4) [4] underlines the
central role of Tyr380 (Fig. 1). Similarly, maltohepta-
ose in the inactive catalytic nucleophile mutant D180A
AMY1 curved with five visible rings around Tyr380.
Two adjacent rings, in a second maltoheptaose mole-
cule with five clearly defined rings, were stacked onto
the indole side chains of Trp278Trp279 on the surface
Fig. 1. Close-up view on the ‘pair of sugar tongs’ binding site in
the crystal structure of a-amylase 1 (AMY1) D180A, an inactive cat-
alytic nucleophile mutant, in complex with maltoheptaose [21].
Important residues defining this site have been highlighted. Ser378
and Tyr380 are mutated in the present work. As continuous elec-
tron density was only found for five sugar rings, a pentasaccharide
was modeled into the structure.
a-amylase ‘sugar tongs’ mutants S. Bozonnet et al.
5056 FEBS Journal 274 (2007) 5055–5067 ª2007 The Authors Journal compilation ª2007 FEBS
of domain A [21]. Seven rings in a third maltoheptaose
molecule occupied subsites )7 through )1 in the active
site [21]. Noticeably, AMY2 accommodated the
pseudotetrasaccharide inhibitor acarbose both at
Trp276Trp277 and at the active site, but not at the
‘sugar tongs’ [3]. Comparison of AMY1 and AMY2
structures [3,4] suggested that Pro376AMY2 – corre-
sponding to Ser378AMY1 – rigidified the loop carry-
ing Tyr378
AMY2
(Tyr380 in AMY1), hindering the
conformational shift needed in oligosaccharide binding
[4]. Different secondary carbohydrate-binding sites are
found in GH-H members, e.g. certain a-amylases
[3,4,22,26–28], cyclodextrin glucosyltransferase [29],
amylosucrase [30], amylomaltase [20], and Thermoacti-
nomyces vulgaris I amylase [31]. The Pseudomonas
maltotetraose-forming amylase structure closely resem-
bles that of AMY1 but has no tyrosine at the position
of Tyr380 [4]. Tyr380, however, is present in several
plant a-amylases [32–34], including AMY2, which did
not accommodate oligosaccharide at the ‘sugar tongs’
in the structure [3]. In the present work, the ‘sugar
tongs’ site was demonstrated by site-directed mutagen-
esis of Tyr380 to be involved in enzymatic activity and
confirmed to be particularly important for carbohy-
drate binding. However, mutating Ser378 in AMY1 to
proline to mimic AMY2 did not elicit lack of binding
as observed for the AMY2 structure [3]. The func-
tional analysis of the surface site furthermore indicated
a role in multivalent binding during polysaccharide
processing.
Results
Choice and production of AMY1 ‘sugar tongs’
mutants
Tyr380 in the ‘sugar tongs’ site on domain C of
AMY1 (Fig. 1) shifted 3.1 A
˚when binding a malto-
oligosaccharide [4,21] and the Y380A, Y380M, and
Y380F enzymes were produced to investigate the
importance of the aromatic side chain, tryptophan
being omitted for steric reasons. The substituted methi-
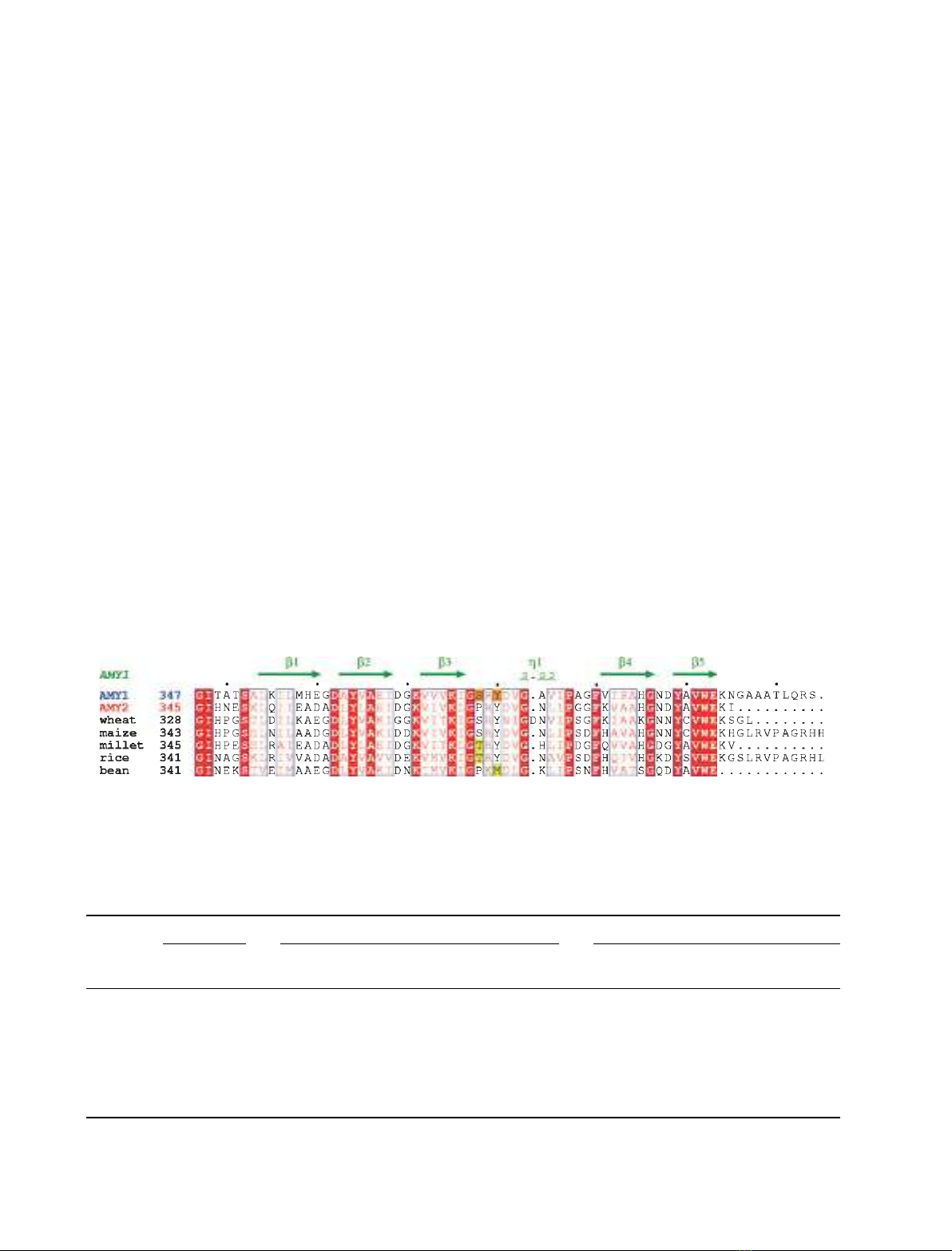
onine also represented a bean a-amylase [35] (Fig. 2).
The lack of sugar binding at the conserved Tyr378 in
the AMY2 ⁄acarbose structure [3] was proposed to be
due to lower mobility imposed by Pro376AMY2 (cor-
responding to Ser378AMY1, see Figs 1 and 2) on the
Arg377–Phe388AMY2 loop. Hence the AMY2 mimic,
AMY1 S378P, was constructed to check the impact of
proline; S378T represented rice and millet a-amylases
[33] (Fig. 2). The host Pichia pastoris secreted
10–44 mgÆL
)1
wild-type and AMY1 mutants as esti-
mated from specific activities against insoluble
blue starch (iBS) of the purified enzymes (Table 1).
Fig. 2. Sequence alignment of domain-C of barley AMY1 and AMY2, four other cereal amylases, and a legume a-amylase. The secondary
structure of AMY1 is indicated above the alignment and mutated residues are highlighted in orange. Accession numbers are; wheat (AMY3):
P08117; maize: Q41770; millet: Q7Y1C3; rice (AMY3): P27933; kidney bean: Q9ZP43.
Table 1. Enzymatic properties of ‘sugar tongs’ mutants of barley a-amylase 1 (AMY1). U, one enzyme unit is the amount required to cause
an A
620
increase of 1.
Enzyme
iBS Amylose DP440 Cl-pNPG
7
Specific activity
(UÆmg
)1
)
k
cat
(s
)1
)
k
m
(mgÆmL
)1
)
k
cat
⁄K
m
(s
)1
mL
)1
Æmg
)1
)
k
cat
(s
)1
)
K
m
(mM)
k
cat
⁄K
m
(s
)1
mM
)1
)
Y380A 1400 95 ± 15 0.363 ± 0.023 261.7 19 ± 0.6 0.669 ± 0.046 28.4
Y380M 2000 149 ± 44 0.351 ± 0.083 424.5 34 ± 0.8 0.871 ± 0.027 39.0
Y380F 2790 162 ± 27 0.391 ± 0.146 414.3 56 ± 1.7 0.724 ± 0.123 77.3
S378P 2695 163 ± 36 0.203 ± 0.130 802.9 59 ± 0.6 0.861 ± 0.023 68.5
S378T 2705 144 ± 9 0.208 ± 0.058 692.3 48 ± 1.7 0.735 ± 0.087 65.3
AMY1 2500 185 ± 20 0.190 ± 0.010 973.7 52 ± 4.9 0.758 ± 0.112 68.6
AMY2 4000 721 ± 63 1.074 ± 0.283 671.3 86 ± 3.1 2.125 ± 0.180 40.5
S. Bozonnet et al. a-amylase ‘sugar tongs’ mutants
FEBS Journal 274 (2007) 5055–5067 ª2007 The Authors Journal compilation ª2007 FEBS 5057
Similarly to the AMY1 wild-type, S378P, S378T and
Y380F were obtained in 50% yield by affinity chro-
matography on b-cyclodextrin (b-CD)-Sepharose,
whereas Y380A and Y380M AMY1 did not bind to
the resin and were purified in 20% yield by ammo-
nium sulfate precipitation and ion exchange chroma-
tography (see Experimental procedures).
Enzymatic activity of ‘sugar tongs’ AMY1
mutants
Replacement of Tyr380 by alanine and methionine
caused 50–75% reduction in the activity of iBS (k
cat
),
amylose DP440 (k
cat
⁄K
m
), and even the oligosaccha-
ride Cl-pNPG
7
(k
cat
⁄K
m
) (Table 1). The mutations
reduced k
cat
for amylose and Cl-pNPG
7
and doubled
K
m
, whereas the conservative substitutions in Y380F,
S378P, and S378T had no effect on enzyme kinetic
parameters except for a twofold increase in K
m
for
Y380F against the amylose (Table 1). This probably
reflected that the mutant was unable to form the
hydrogen bond between Tyr380 cOH and O2 of glu-
cose as seen in the AMY1Æthio-DP4 complex [4]. Activ-
ity for iBS was routinely analyzed under saturating
conditions (i.e. 6.25 mgÆmL
)1
iBS), but in fact AMY1
showed a small and highly reproducible isozyme-char-
acteristic activity maximum near 2 mgÆmL
)1
iBS corre-
sponding to 115% of the activity at 6.25 mgÆmL
)1
iBS.
This property was lost in Y380A, suppressed for
Y380M, but retained by Y380F, S378P, and S378T
AMYl, and was missing for AMY2 (data not shown).
The earlier reported hydrolysis of the amylose of
DP440 in a multiple attack mechanism [12] was con-
firmed for AMY1, which showed a DMA of 1.9 as
determined from the ratio of rates of release of
reducing groups in the fraction of small (i.e. ethanol-
soluble) products over large (i.e. ethanol-precipitated)
products (see Experimental procedures and [12]). The
rates of product formation by the mutants (not
shown) agreed with the activity levels described in
Table 1. AMY1 Y380A had a DMA of 1.0 and thus
released fewer short products per enzyme–substrate
encounter than AMY1 wild-type, whereas AMY1
Y380M and S378P maintained a DMA of 2.0 and
2.2, respectively.
Binding of b-cyclodextrin to ‘sugar tongs’
mutants measured by surface plasmon resonance
analysis
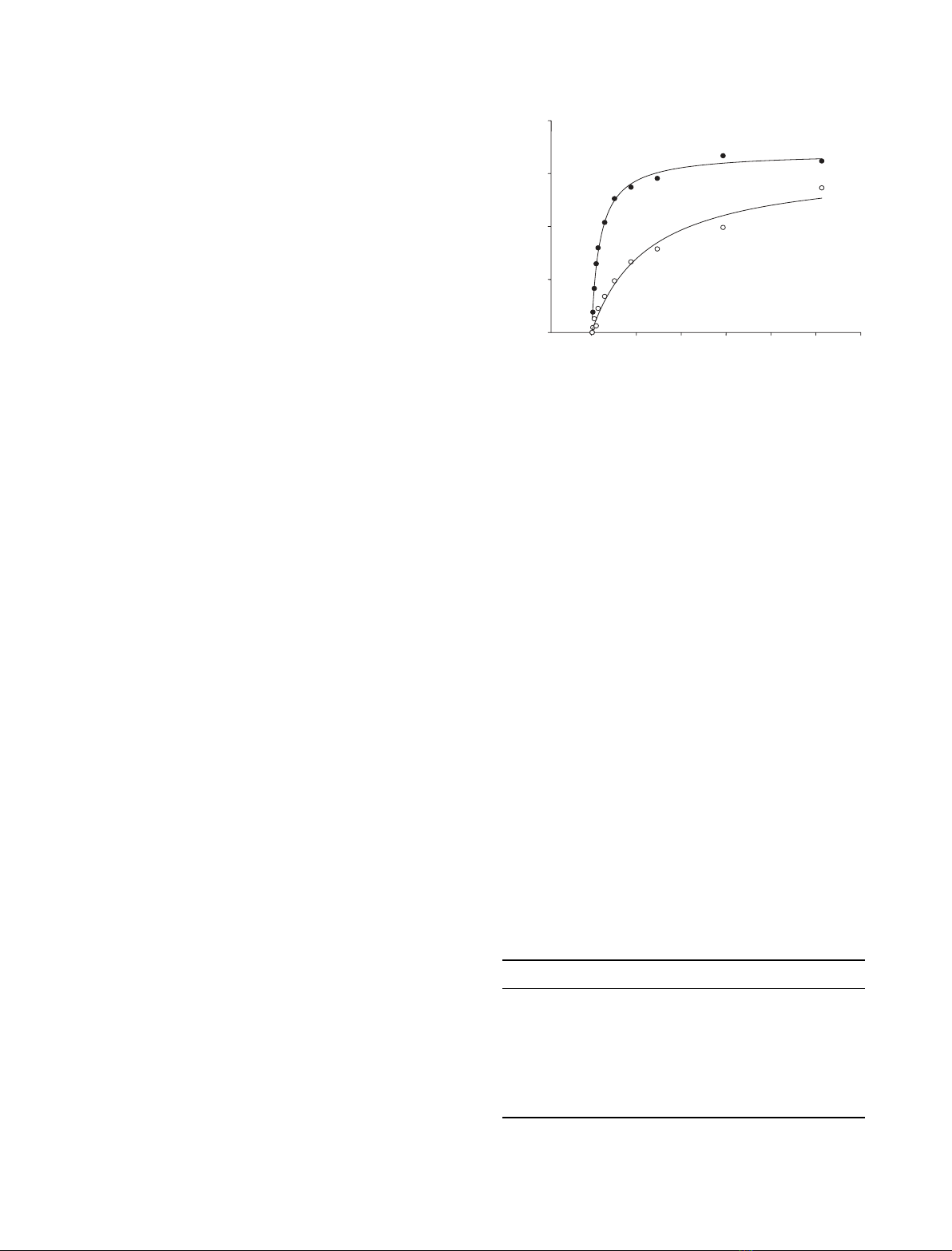
Surface plasmon resonance (SPR) analysis was suitable
for measuring the affinity in the low millimolar range
of b-CD for AMY1. SPR sensorgrams clearly illustrated
weaker binding to AMY1 Y380A than to wild-type
enzyme (Fig. 3) and K
d
was calculated to 1.40 mmfor
both Y380A and Y380M, i.e. sevenfold higher than K
d
of AMY1 wild-type (Table 2). Y380F caused only a
slight reduction in affinity for b-CD and the binding to
S378P and S378T was essentially not affected by the
mutations. In comparison, the K
d
of AMY2 was three-
fold higher than that of AMY1 (Table 2).
Effects of ‘sugar tongs’ mutation on adsorption
to and hydrolysis of starch granules
Starch granules are the natural substrate for barley
a-amylases and it was hypothesized that the ‘sugar
tongs’ might play a role in interaction with this sub-
strate of giant size compared to the enzyme. The adsorp-
tion to barley starch granules of ‘sugar tongs’ mutants
was therefore examined. The K
d
was 0.47 mgÆmL
)1
for
AMY1 wild-type and very similar for S378P, but 13-fold
higher for AMY1 Y380A (Table 3). This indication of a
β
β
-cyclodextrin (mM)
01 34526
RU
0
100
200
300
400
Fig. 3. b-CD binding determined by SPR analysis. AMY1: dwild-
type, sY380A. Response unit (RU) values are corrected for the
contribution given by a channel in the chip without bound enzyme
protein.
Table 2. Binding of b-cyclodextrin (b-CD) to ‘sugar tongs’ mutants
and wild-type AMY1 and AMY2 as determined by SPR. See Experi-
mental procedures for the SPR analytical procedure.
Enzyme K
d
(mM)
Y380A 1.40 ± 0.23
Y380M 1.39 ± 0.65
Y380F 0.36 ± 0.02
S378P 0.25 ± 0.03
S378T 0.23 ± 0.02
AMY1 0.20 ± 0.04
AMY2 0.63 ± 0.27
a-amylase ‘sugar tongs’ mutants S. Bozonnet et al.
5058 FEBS Journal 274 (2007) 5055–5067 ª2007 The Authors Journal compilation ª2007 FEBS
very important role of Tyr380 is in accordance with
b-CD having no impact on the apparent affinity of
AMY1 Y380A for starch granules, whereas the presence
of 0.5 mmb-CD increased the apparent K
d
four- to six-
fold for AMY1 wild-type and S378P and AMY2
(Table 3), confirming competition in binding to starch
granules. AMY2 showed threefold weaker affinity for
barley starch granules than did both the wild-type and
the AMY2 mimic, AMY1 S378P (Table 3).
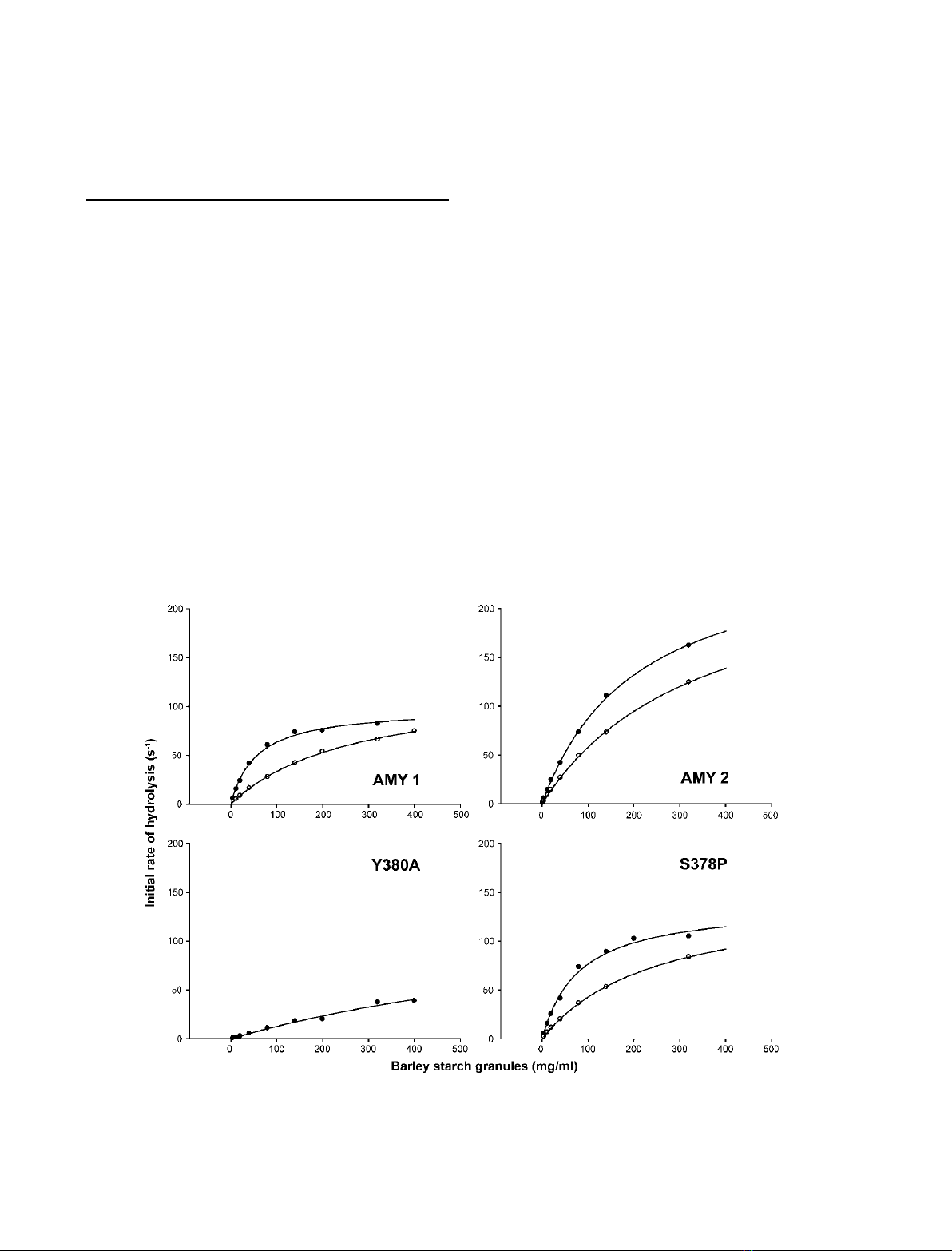
The ‘sugar tongs’ substitution in AMY1 Y380A
greatly influenced the hydrolytic activity against granu-
lar starch, with release of soluble reducing sugars from
this substrate being strongly reduced (Fig. 4) to a
k
cat
⁄K
m
value of 10% of that of AMY1 wild-type. In
contrast to wild-type, substrate saturation was not
achieved for AMY1 Y380A even at 400 mgÆmL
)1
of
starch granules and the shape of the corresponding
activity curve indicated that loss in substrate affinity
was a predominant factor in the reduced activity
(Fig. 4). For AMY1 S378P, k
cat
and K
m
were similar
to the wild-type values (Table 4), but AMY2 had infe-
rior affinity. The corresponding activity curve (Fig. 4)
allowed only estimation of kinetic parameters, the K
m
being considerably higher than in the case of AMY1,
whereas the k
cat
for AMY2 appeared higher than for
AMY1, as found in general for different substrates
(Table 1). The activity was reduced in the presence of
b-CD (Fig. 4; Table 4) due to competition with starch
granule binding. The low activity hampered analysis of
the effect of b-CD on AMY1 Y380A.
Table 3. Binding of ‘sugar tongs’ mutants, wild-type AMY1 and
AMY2 to barley starch granules. The binding was measured in the
range 0.01–40 mgÆmL
)1
starch granules (see Experimental proce-
dures and [29] for details). (A) no b-CD; (B) in the presence of
0.5 mMb-CD.
Enzyme K
d
(mgÆmL
)1
)B
max
A
Y380A 5.90 ± 0.47 0.90 ± 0.05
S378P 0.57 ± 0.04 0.98 ± 0.01
AMY1 0.47 ± 0.06 1.03 ± 0.04
AMY2 1.27 ± 0.32 0.99 ± 0.03
B
Y380A 6.86 ± 0.55 0.81 ± 0.02
S378P 2.93 ± 0.36 0.95 ± 0.02
AMY1 2.85 ± 0.28 0.98 ± 0.02
AMY2 4.63 ± 0.37 0.87 ± 0.02
Fig. 4. Rates of release of soluble reducing products from barley starch granules as catalyzed by AMY1, AMY2, and Y380A and
S378P AMY1 in the absence (d), and in the presence (s) of 0.5 mMb-CD.
S. Bozonnet et al. a-amylase ‘sugar tongs’ mutants
FEBS Journal 274 (2007) 5055–5067 ª2007 The Authors Journal compilation ª2007 FEBS 5059
















![Báo cáo seminar chuyên ngành Công nghệ hóa học và thực phẩm [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250711/hienkelvinzoi@gmail.com/135x160/47051752458701.jpg)









