
Dimerization and oligomerization of the chaperone calreticulin
Charlotte S. Jørgensen
1
, L. Rebekka Ryder
1
, Anne Steinø
1
, Peter Højrup
2
, Jesper Hansen
2
, N. Helena Beyer
3
,
Niels H. H. Heegaard
3
and Gunnar Houen
1
1
Department of Research and Development, Statens Serum Institut, Copenhagen, Denmark;
2
Department of Biochemistry and
Molecular Biology, University of Southern Denmark, Odense, Denmark;
3
Department of Autoimmunology,
Statens Serum Institut, Copenhagen, Denmark
The chaperone calreticulin is a highly conserved eukaryotic
protein mainly located in the endoplasmic reticulum. It
contains a free cysteine SH group but does not form disul-
fide-bridged dimers under physiological conditions, indica-
ting that the SH group may not be fully accessible in the
native protein. Using PAGE, urea gradient gel electro-
phoresis, capillary electrophoresis and MS, we show that
dimerization through the SH group can be induced by
lowering the pH to 5–6, heating, or under conditions that
favour partial unfolding such as urea concentrations above
2.6
M
or SDS concentrations above 0.025%. Moreover, we
show that calreticulin also has the ability to self-oligomerize
through noncovalent interactions at urea concentrations
above 2.6
M
at pH below 4.6 or above pH 10, at tempera-
tures above 40 C, or in the presence of high concentrations
of organic solvents (25%), conditions that favour partial
unfolding or an intramolecular local conformational change
that allows oligomerization, resulting in a heterogeneous
mixture of oligomers consisting of up to 10 calreticulin
monomers. The oligomeric calreticulin was very stable, but
oligomerization was partially reversed by addition of 8
M
urea or 1% SDS, and heat-induced oligomerization could
be inhibited by 8
M
urea or 1% SDS when present during
heating. Comparison of the binding properties of mono-
meric and oligomeric calreticulin in solid-phase assays
showed increased binding to peptides and denatured pro-
teins when calreticulin was oligomerized. Thus, calreticulin
shares the ability to self-oligomerize with other important
chaperones such as GRP94 and HSP90, a property possibly
associated with their chaperone activity.
Keywords: calreticulin; chaperone; dimerization; heat shock
protein; oligomerization.
Calreticulin is a highly conserved ubiquitous protein, mainly
located in the endoplasmic reticulum [1,2]. It has been found
to be involved in many cellular processes, including calcium
storage and chaperone function, and it has been reported to
possess carbohydrate and peptide binding properties, and to
play a role in assembly of the MHC I loading complex
[1–9]. The crystal structure of the lumenal domain of
the homologous membrane-bound chaperone calnexin has
revealed a protein with a compact globular N domain with
homology to legume lectins, composed of two antiparallel
b-sheets and a long P domain bhairpin arm stretching away
from the globular domain (Fig. 1) [10,11]. A calreticulin
model has been proposed based on the calnexin structure,
suggesting a globular N domain consisting of a concave and
a convex b-sheet, a P domain composed of two antiparallel
b-strands shown by NMR to form an extended hairpin fold,
and a C domain with b-sheet and a-helical structure in the
first part, proposed to shield the hydrophobic regions of the
convex b-sheet, and random-coil structure in the second
half [7,12–15]. In accordance with the calreticulin model,
proteolytic mapping studies of calreticulin have shown that
proteolytic cleavage with various proteases generates a
truncated form lacking a major part of the C domain,
confirming the presence of a looser structure in the second
half of the C domain [15–18].
Previous studies ([15,17]; C. S. Jørgensen, C. Trandum,
L. R. Ryder, M. Gajhede, L. K. Skov, P. Højrup,
V. Bakholt & G. Houen, unpublished results) have shown
that calreticulin has a rather low T
m
, which is surprising as it
is a heat shock protein. Furthermore, it was found to be a
conformationally flexible protein. These properties may be
related to its function as a chaperone and stress protein, and
therefore we decided to investigate the protein further in
response to various forms of physical stress. We subjected
it to high and low pH, elevated temperatures, or high
concentrations of urea, detergent, or organic solvent, and
recorded the behaviour of the protein using PAGE, MS,
and capillary electrophoresis. Calreticulin responded by
dimerizing and oligomerizing. Oligomerization is a property
that it shares with other important chaperones such as
GRP94 and HSP90 [19–21], and it is possibly associated
with its chaperone function.
Materials and methods
Materials
Glycine, Tris, Bistris, dithiothreitol, formaldehyde, silver
nitrate, Triton X-114, Triton X-100, glycerol, urea, EDTA,
diethanolamine, p-nitrophenyl phosphate, bromophenol
Correspondence to G. Houen, Department of Research and Devel-
opment, Statens Serum Institut, Artillerivej 5, 2300 Copenhagen S,
Denmark. Fax: + 45 32683149, Tel.: + 45 32683276,
E-mail: gh@ssi.dk
(Received 29 April 2003, revised 1 August 2003,
accepted 28 August 2003)
Eur. J. Biochem. 270, 4140–4148 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03808.x

blue, MgCl
2
, ZnCl
2
, fucose, galactose, b-lactoglobulin,
ovalbumin, alkaline phosphatase-conjugated goat immuno-
globulins against rabbit/mouse immunoglobulins, and
sinnapinic acid were from Sigma (St Louis, MO, USA).
NaCl, CaCl
2
, acetic acid, NaHCO
3
, Na
2
CO
3
(NH
4
)
2
SO
4
,
sodium thiosulfate, glucose, dimethylformamide, dimethyl
sulfoxide, and Tween 20 were from Merck (Darmstadt,
Germany). Acetonitrile and trifluoroacetic acid were from
Rathburn (Walkerburn, Scotland, UK). Mouse monoclo-
nal antibody against calreticulin was from Stressgen (Vic-
toria, British Columbia, Canada). Mannose was from
Fluka (Buchs, Switzerland). Tris/glycine gels (4–20/4–
12%) were from Novex (San Diego, CA, USA). Ethanol
was from Danisco (Aalborg, Denmark). SDS was from
BDH (Poole, Dorset, UK). Acrylamide and bisacrylamide
were from SSI Diagnostika (Hillerød, Denmark). Q Seph-
arose Fast Flow and Sephacryl S-100 were from Pharmacia
(Uppsala, Sweden). Poros 50 R1 was from Applied
Biosystems (Foster City, CA, USA). Milli Q water equip-
ment, 10-kDa ultrafilters, and Centriprep centrifuge tubes
(10-kDa cutoff) were from Millipore (Bedford, MA, USA).
Maxisorp microtiter ELISA plates were from Nunc (Ros-
kilde, Denmark). Rabbit antisera against calreticulin were
prepared as described previously [22].
Purification of human placental calreticulin
Human placental calreticulin was purified and identified
using minor modifications of a well-established procedure
[18]: 20 m
M
Bistris, pH 7.2, was used as buffer instead of
sodium phosphate; the second ammonium sulfate precipi-
tation was not performed, but instead an ultradiafiltration
against 20 m
M
Tris/HCl, pH 7.5, followed by Q Sepharose
ion-exchange chromatography using 20 m
M
Tris/HCl,
pH 7.5, as buffer with stepwise elution using increasing
concentrations of NaCl in the same buffer. Fractions
containing calreticulin were identified by SDS/PAGE and
ELISA using antisera that recognize the N-termini and
C-termini of calreticulin [22], pooled and concentrated by
ultradiafiltration against 20 m
M
Tris/HCl, pH 7.5, followed
by size-exclusion chromatography on a Sephacryl S-100 HR
column. The protein showed a single band of apparent
molecular mass 60 kDa on SDS/PAGE and a single band
of pI 4.6 on isoelectric focusing.
Native PAGE
Samples were mixed with an equal volume of sample buffer
(0.2
M
Tris/HCl, pH 8.8, 10% glycerol, 0.005% bromo-
phenol blue), and loaded on 4–20% or 4–12% Tris/glycine
gels (Novex). Electrophoresis was carried out at 150 V for
75 min using 25 m
M
Tris/192 m
M
glycine, pH 8.5, as
electrophoresis buffer. After the electrophoresis, the
gels were silver stained using the procedure described by
Blum et al. [23].
Urea gradient PAGE
Electrophoretic analysis of protein folding across a trans-
verse urea gradient was carried out as described by
Creighton [24–26] using 11% polyacrylamide gels. A Novex
gel-moulding cassette was modified to facilitate casting of
the gels. The urea gradients (0–8
M
or 1–7
M
)weremadein
50 m
M
Tris/HCl (pH 8.8)/11% acrylamide/0.3% bisacryl-
amide, with two chambers connected. The mixing chamber
was stirred with a magnetic bar, and a peristaltic pump was
used to fill the gel cassettes. Samples were incubated at room
temperature for 1 h in the presence or absence of 8
M
urea
and 5 m
M
dithiothreitol. Glycerol and bromophenol blue
were then added to final concentrations of 10% and 0.1%.
The gels were run at 4 C overnight at 40 V using 50 m
M
Tris/HCl, pH 8.0, as buffer.
Digestion of heat-denatured ovalbumin
Denaturation and proteolytic digestion of heat-denatured
ovalbumin was performed as described in Jørgensen et al.
[6].
ELISA
A proteinase K digest of heat-denatured ovalbumin, a
peptide (GYVIIKPLVWV [6]), or ovalbumin (1 mgÆmL
)1
)
was diluted 1 : 10/1 : 500/1 : 1000 followed by overnight
incubation at 5 C; 100 lL per well using 50 m
M
Na
2
CO
3
,
pH 9.6, with or without the addition of 8
M
urea/50 m
M
dithiothreitol as coating buffer. All subsequent incubations
and washing steps were in 25 m
M
Tris/HCl (pH 7.5)/0.15
M
NaCl/0.5% Tween 20. The plate was washed three times for
1 min, followed by a 30-min blocking step using the same
buffer. The wells were incubated for 2 h with calreticulin
(0.25 mgÆmL
)1
, diluted 1 : 200) or heat-treated calreticulin
(1 h at 57 C; diluted 1 : 200). After being washed
(3 ·1 min), the plate was incubated for 1 h with monoclo-
nal antibody against calreticulin, washed again (3 ·1min)
and incubated for 1 h with alkaline phosphatase-conjugated
goat immunoglobulins against mouse immunoglobulins.
After another three washes, bound conjugate was quantified
using a p-nitrophenyl phosphate solution (1 mg p-nitro-
phenyl phosphate per mL of 1
M
diethanolamine, pH 9.8,
0.5 m
M
MgCl
2
). The plate was read on a VERSAmax
turnable microplate reader (Molecular Devises, Sunnyvale,
CA, USA) at 405 nm using background subtraction at
690 nm.
Fig. 1. Crystal structure of the lumenal domain of calnexin. 1JHN in
The Protein Data Bank found at http://www.rcsb.org/pdb/ [10,39].
FEBS 2003 Dimerization and oligomerization of calreticulin (Eur. J. Biochem. 270) 4141

MALDI-TOF-MS
Protein micropurification and sample application was
performed as described previously [27], using Poros 50 R1
for the micropurification. Samples were eluted with matrix
(20 lgÆlL
)1
sinnapinic acid in 70% acetonitrile/0.1%
trifluoroacetic acid) directly on to the first matrix layer
(20 lgÆlL
)1
sinnapinic acid in 100% acetone) on the target
plate [Scout 384 massive (aluminium) from Bruker Dalton-
ics, Bremen, Germany].
Delayed extraction MALDI-TOF MS was carried out on
a Bruker ultraflex MALDI reflector time-of-flight mass
spectrometer (Bruker Daltonics) equipped with a nitrogen
laser (k¼337 nm). All mass spectra were collected in the
linear positive ion mode. External calibration was carried
out with protein standard II from Bruker (Bruker Dalton-
ics). Data analysis was carried out using either the
M/Z
software package (
M
/
Z
-Freeware edition, 2001-08-14; Pro-
teometrics Inc., New York, NY, USA) or
XTOF
1.5 (Bruker
Daltonics).
Capillary electrophoresis
Capillary electrophoresis was performed on a Beckman
P/ACE 2050 instrument using UV detection at 200 nm.
Electrophoresis buffer was 0.1
M
phosphate, pH 7.4. A
50-lm internal diameter uncoated fused silica capillary with
50 cm to the detector window and of 57 cm total length was
used. Separations were carried out at a constant current of
80 lA (corresponding to voltages of 18 kV). The capil-
lary was thermostatically controlled at 20 C. Data were
collected and processed by the Beckman system Gold
software. The capillary was rinsed after electrophoresis for
1minwith0.1
M
NaOHand1minwithwaterandthenfor
2 min with electrophoresis buffer. Samples for the heating
experiments consisted of calreticulin at 0.20 mgÆmL
)1
in
NaCl/P
i
mixed with a peptide marker (Ac-Pro-Ser-Lys-Asp-
OH)at0.1 mgÆmL
)1
in a final volume of 50 lL. Then 30 lL
of the sample was heated at 48 C in an Eppendorf
thermomixer (500 r.p.m.). At 20, 70 and 110 min, 10 lL
aliquots were withdrawn and kept at )20 C until analysed
by capillary electrophoresis. The capillary electrophoresis
analysis of the aliquots subsequently took place after
dilution with 5 lL water and injection for 6 s corresponding
to 5 nL sample volumes.
Results
Dimerization of calreticulin
Calreticulin contains three cysteines, of which the first two
(Cys88, Cys120) form a disulfide bridge whereas the third
(Cys146) is free [18]. Initially we evaluated the accessibility
of the cysteine side chains in calreticulin to thiol-specific
reagents using MS. As expected, Cys146 reacted readily
with the small molecule iodoacetic acid, whereas Cys88 and
Cys120 were not derivatized (results not shown). This
confirms that calreticulin has a free SH group on Cys146. In
purified human placenta calreticulin, dimers were absent but
we found that dimerization could be induced experiment-
ally. Lowering the pH from 7 to 6 or 5 resulted in the
appearance of a higher-molecular-mass band in native
PAGE, with a mobility corresponding to a calreticulin
dimer (Fig. 2). The band was identified as calreticulin by
immunoblotting using a rabbit antiserum against the
C-terminus of calreticulin, and as a covalently linked dimer
from the molecular mass determined by MS analysis (results
not shown). Dimerization was also seen in native PAGE
after exposure of calreticulin to urea (above 2.6
M
)orto
SDS (at or above 0.025%). Moreover, urea also induced a
small amount of oligomerization of calreticulin, which will
be addressed in the next section. Dimerization by exposure
to urea was further demonstrated by electrophoretic ana-
lysis of calreticulin unfolding and refolding across a gradient
of urea in polyacrylamide gels (Creighton gels; Fig. 3).
When calreticulin was applied in native form and subjected
to electrophoresis in the urea gradient gel, the occurrence of
a dimer, formed at urea concentrations 3
M
, could be seen
to correlate roughly with the occurrence of the unfolded
form of the protein. When calreticulin was applied in 8
M
urea, the dimer was present throughout the gel. These
results show that the free SH group on Cys146 in calreticulin
is incapable of dimerization in the native conformation of
calreticulin, but that it becomes exposed and capable of
dimerization under conditions favouring partial or complete
unfolding of the protein.
Oligomerization of calreticulin
As mentioned above, besides induction of dimerization,
2.7
M
urea also induced a small degree of oligomerization
of calreticulin. This effect was also seen at higher urea
concentrations and was maximal at 5–6
M
urea. At higher
urea concentrations (7–8
M
), the larger oligomers were
absent but trimers and tetramers were present in addition to
the dimer (results not shown). Recombinant calreticulin has
been reported to oligomerize/polymerize at 37–45 C[28],
and in agreement with this we could also demonstrate a
temperature-dependent oligomerization of purified human
placenta calreticulin. As seen in Fig. 4A, oligomerization
was observed when the temperature was raised from 37 C
to 47 C, and even more pronounced at 57 Cand67C.
Fig. 2. Silver-stained native PAGE analysis (4–12 Tris/glycine gel) of
pH-induced dimerization of calreticulin. Calreticulin was dialysed
against 20 m
M
Tris/HCl, pH 5, 6, 7, or 8, as indicated below the gel.
The calreticulin preparation used shows one major and two minor
calreticulin bands just above and below the major band. Immuno-
blotting experiments and MS analysis confirmed that all three bands
contained calreticulin (results not shown).
4142 C. S. Jørgensen et al.(Eur. J. Biochem. 270)FEBS 2003
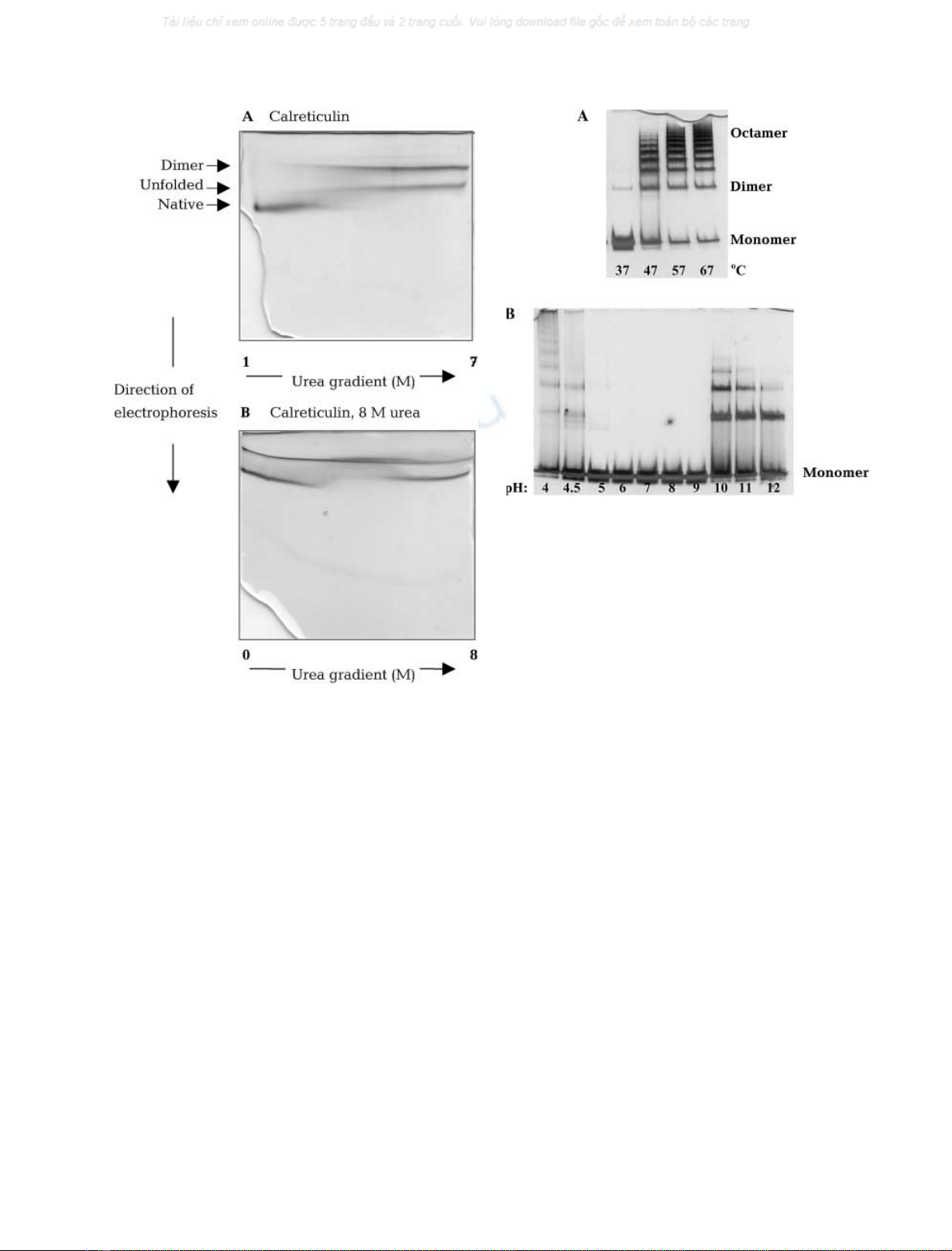
The oligomerization temperature, defined as the tempera-
ture at which higher-molecular-mass bands began to appear
upon native PAGE, was determined to be 40 C. A new
result was the finding that lowering the pH below 4.6 or
increasing pH above 10 also induced oligomerization
(Fig. 4B), as did the presence of 25% organic solvents
(dimethylsulfoxide, dimethylformamide, ethanol or meth-
anol) and nonionic detergent (Tween 20) (data not shown).
Investigation of the temperature-dependent oligomerization
at 47 C showed that it was a relatively fast reaction, with
oligomers observed after 10 min and maximal oligomeriza-
tion after 1–2 h (results not shown). Most of the oligomers
appeared, by visual inspection of native polyacrylamide
gels, to consist of dimers to octamers, but larger oligomers
were also observed. The identity of the higher-molecular-
mass bands was confirmed by immunoblotting using an
antibody against the C-terminal part of calreticulin (results
not shown). Visual inspection of the silver-stained PAGE
gel confirmed that the calreticulin band (monomer) was
actually decreasing in intensity as the higher-molecular-
mass bands appeared. Control experiments were performed
with other proteins with low pI values (human serum
albumin, pI 4.9; ovalbumin, pI 5.2; b-lactoglobulin, pI 5.2).
These were tested for their ability to oligomerize at low pH
or elevated temperatures, but none oligomerized, confirm-
ing that oligomerization induced by pH or temperature is
not a general property of proteins with low pI values, but a
specific feature of selected proteins including calreticulin.
Raising the pH to 7 after pH-induced oligomerization, or
lowering the temperature after temperature-induced oligo-
merization did not reverse the effect, indicating that once
formed the calreticulin oligomers were stable (results not
shown).
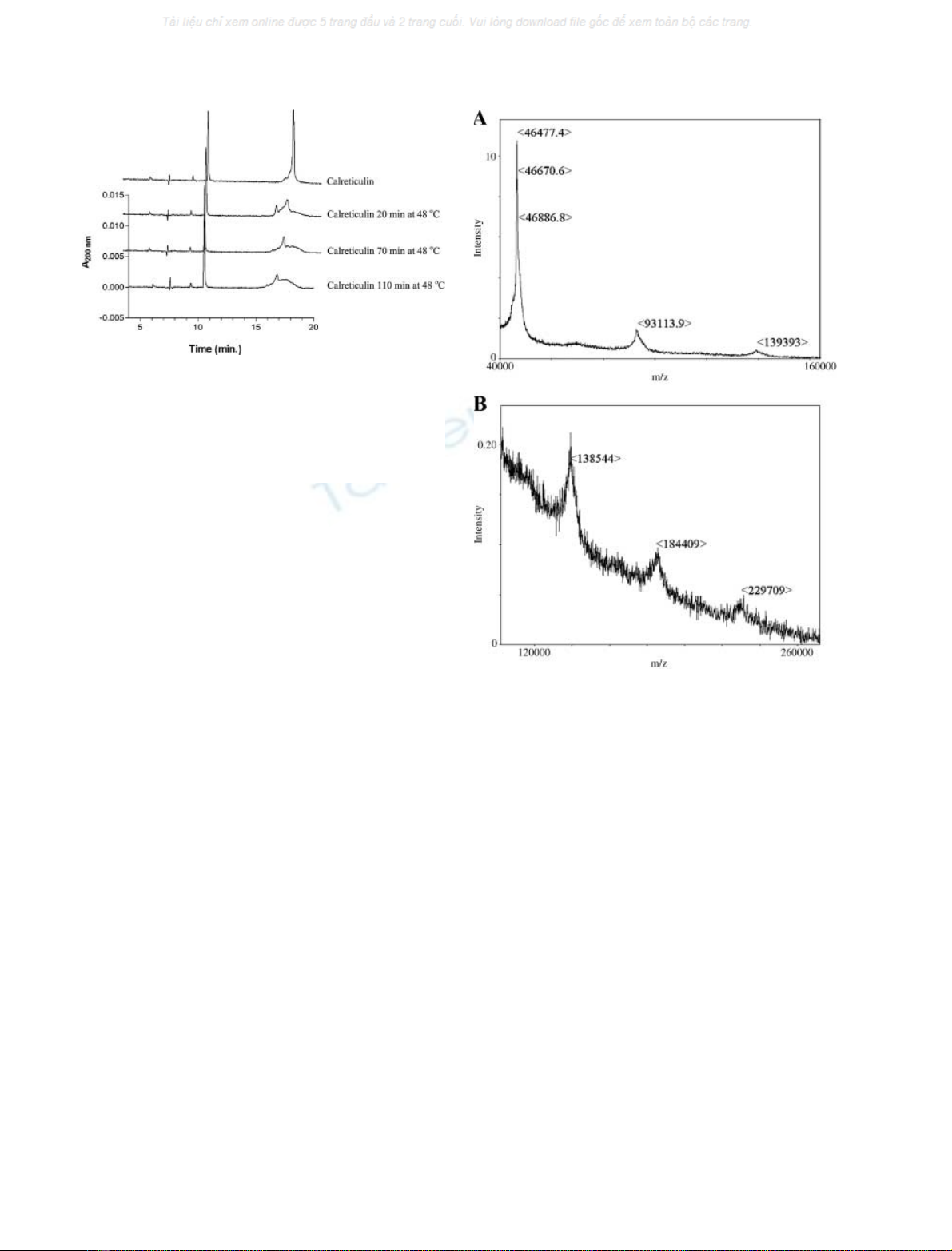
Capillary electrophoresis of heat-treated calreticulin
compared with nontreated calreticulin confirmed that the
peak of the monomeric calreticulin (detected at 18 min)
was reduced on heating: the longer the heating time or the
higher the temperature, the smaller the peak became
(Fig. 5). As capillary electrophoresis separates molecules
according to their mass/charge ratios, the oligomers do not
show up individually in the electropherogram, but form a
broad peak detected with about the same migration time as
the monomer. As a control, a marker peptide (detected at
10 min) was added to the calreticulin sample, and the size
of this peak remained unchanged throughout the experi-
ment, confirming that the reduction in the monomeric
calreticulin band was specific to calreticulin, and not an
experimentally induced artefact.
MALDI-TOF MS analysis (Fig. 6) of heat-treated cal-
reticulin showed peaks corresponding in mass up to at least
pentameric calreticulin, confirming that the heat-treated
Fig. 3. Creighton gels showing urea-induced unfolding and dimerization
of calreticulin. Urea gradient (0–8/1–7
M
) PAGE of calreticulin
(1 mgÆmL
)1
) folding and unfolding in 20 m
M
Tris/HCl, pH 7.5.
(A) Calreticulin loaded on the gel in native form. (B) Calreticulin
loadedafter1hofincubationin8
M
urea. Gels were stained with
Coomassie Brilliant Blue.
Fig. 4. Oligomerization of calreticulin analysed by native PAGE with
silver staining (4–12% Tris/glycine gels). (A) Heat-induced; calreticulin
was incubated for 60 min at 37 C, 47 C, 57 C, or 67 C. (B) pH-
induced; calreticulin was incubated for 90 min at pH values between
4 and 12, as indicated.
FEBS 2003 Dimerization and oligomerization of calreticulin (Eur. J. Biochem. 270) 4143
calreticulin consisted of assemblies of integer numbers of
calreticulin molecules. However, the oligomerization may
be partly induced by the experimental conditions in the
sample sandwich on the MALDI target.
Reduced SDS/PAGE of trypsin-treated (2 h at 37 C)
monomeric and oligomeric calreticulin indicated that the
oligomeric calreticulin was more sensitive to trypsin diges-
tion (Fig. 7). MALDI-TOF MS analysis of the resulting
bands with lower-molecular-mass confirmed that cleavage
had taken place exclusively from the C-terminus of calreti-
culin (main fragments identified: 1–334, 1–261, and 1–205).
The temperature-dependent oligomerization was also
investigated using a truncated form of calreticulin (residues
1–334 [18]). The truncated calreticulin retained the ability to
oligomerize, indicating that the C-terminus of calreticulin
was not essential for oligomerization (Fig. 8).
When oligomerization was induced by heat, pH, or
dimethyl sulfoxide in the presence of 5 m
M
dithiothreitol,
the disulfide-bridged dimer was not observed on native
PAGE, but the larger oligomers appeared (Fig. 9A). This
shows that a disulfide bridge mediates the formation of a
dimer whereas the larger oligomers are formed by a
mechanism not involving disulfide bridges. Oligomerization
in the presence of dithiothreitol must involve formation of a
noncovalent dimer, to which further monomers are added,
but apparently this dimer further oligomerizes. From this it
follows that two mechanisms of dimerization are possible in
the absence of dithiothreitol, one disulfide bridge-mediated,
and one involving only noncovalent interactions. It is
conceivable that the disulfide-bridged dimer can further
oligomerize, but from these results, it appears that the
disulfide-bridged dimer does not oligomerize as easily as the
noncovalent dimer. Exposure of the oligomers to highly
denaturing conditions by addition of 8
M
urea or 1% SDS
after oligomerization of calreticulin resulted in partial
reversal of the oligomerization; the largest oligomers
disappeared but the smaller oligomers and the dimer were
still present (Fig. 9B). In denaturing, reducing SDS/PAGE
analysis of heat-treated calreticulin, oligomeric calreticulin
was reduced to monomeric calreticulin, showing that
heating in the presence of SDS and dithiothreitol could
reverse the dimerization and oligomerization (data not
shown). The presence of either 8
M
urea or 1% SDS during
heat treatment also completely inhibited the oligomerization
of calreticulin, and only the calreticulin dimer was observed
on native PAGE, consistent with the observation that urea
and SDS induces dimerization (Fig. 9B). The oligomeriza-
tion was further investigated in the presence of additives
with the potential to stabilize or destabilize the protein
(12 m
M
CaCl
2
, MgCl
2
, ZnCl
2
, EDTA, fucose, mannose,
glucose, or galactose), and none of these prevented the
oligomerization of calreticulin (results not shown). Obser-
vations by Li et al. [17], who by CD analysis showed that
Ca
2+
acted as a stabilizing ion, increasing thermal stability
[from T
m
¼40.2 CtoT
m
(Ca
2+
)¼44.3–46.4 C, increas-
ing with increasing Ca
2+
concentration], whereas Zn
2+
acted as a destabilizing ion decreasing thermal stability
[T
m
(Zn
2+
)¼29.9–36.7 C, decreasing with increasing
Fig. 6. MALDI-TOF MS of calreticulin demonstrating oligomerization
of calreticulin, heated for 30 min at 50 C. (A) Calreticulin monomer,
dimer and trimer with molecular masses of 46 477, 93 113 and
139 393 Da, respectively. (B) Calreticulin trimer, tetramer and pen-
tamer with molecular masses of 138 544, 184 409 and 229 709 Da,
respectively.
Fig. 5. Time course of changes in heat-treated calreticulin as monitored
by capillary electrophoresis. Four separate analyses are shown of
samples of calreticulin mixed with a peptide marker and exposed to
either room temperature (upper trace) or increasing times at 48 Cas
indicated. Whereas the peptide marker at 10 min is unchanged, there
are marked changes in the calreticulin peak at 18 min upon heating of
the sample.
4144 C. S. Jørgensen et al.(Eur. J. Biochem. 270)FEBS 2003


























