
Calcite-specific coupling protein in barnacle underwater
cement
Youichi Mori
1
, Youhei Urushida
1
, Masahiro Nakano
1
, Susumu Uchiyama
2
and Kei Kamino
1
1 Marine Biotechnology Institute, Kamaishi, Iwate, Japan
2 Department of Biotechnology, Graduate School of Engineering, Osaka University, Japan
Sessile organisms are destined for attachment to vari-
ous materials in water. Because gregariousness is essen-
tial for them, the opportunity to attach to a calcific
exoskeleton of the same kind is necessarily favored.
Thus, calcific material is one of the frequent foreign
materials for attachment in the molecular system of
the holdfast.
The barnacle is a unique sessile crustacean. Once the
larva has settled on the foreign substratum, it metamor-
phoses, calcifying the outer shell at the periphery and
base, and permanently attaches to the foreign substra-
tum by a multiprotein complex called cement [1]. This
cement is secreted through the calcareous base to an
acellular milieu, and joins two different materials, the
Keywords
adsorption; crustacean; protein complex;
sessile organism; underwater adhesive
Correspondence
K. Kamino, Marine Biotechnology Institute,
3-75-1 Heita, Kamaishi, Iwate 026-0001
Japan
Fax: +81 193 26 6592
Tel.: +81 193 26 6584
E-mail: kei.kamino@mbio.jp
Database
The nucleotide sequence data are available
in the DNA Data Bank of Japan under the
accession number AB329666
(Received 5 July 2007, revised 18 October
2007, accepted 23 October 2007)
doi:10.1111/j.1742-4658.2007.06161.x
The barnacle relies for its attachment to underwater foreign substrata on
the formation of a multiprotein complex called cement. The 20 kDa cement
protein is a component of Megabalanus rosa cement, although its specific
function in underwater attachment has not, until now, been known. The
recombinant form of the protein expressed in bacteria was purified in solu-
ble form under physiological conditions, and confirmed to retain almost
the same structure as that of the native protein. Both the protein from the
adhesive layer of the barnacle and the recombinant protein were character-
ized. This revealed that abundant Cys residues, which accounted for 17%
of the total residues, were in the intramolecular disulfide form, and were
essential for the proper folding of the monomeric protein structure. The
recombinant protein was adsorbed to calcite and metal oxides in seawater,
but not to glass and synthetic polymers. The adsorption isotherm for
adsorption to calcite fitted the Langmuir model well, indicating that the
protein is a calcite-specific adsorbent. An evaluation of the distribution of
the molecular size in solution by analytical ultracentrifugation indicated
that the recombinant protein exists as a monomer in 100 mmto 1 mNaCl
solution; thus, the protein acts as a monomer when interacting with the
calcite surface. cDNA encoding a homologous protein was isolated from
Balanus albicostatus, and its derived amino acid sequence was compared
with that from M. rosa. Calcite is the major constituent in both the shell of
barnacle base and the periphery, which is also a possible target for the
cement, due to the gregarious nature of the organisms. The specificity of
the protein for calcite may be related to the fact that calcite is the most
frequent material attached by the cement.
Abbreviations
ASW, artificial seawater; C
eq
, equilibrium protein concentration; C
I
, initial protein concentration; cp, cement protein; fp, mussel foot protein;
GSF1 and GSF2, cement fractions separated by their solubility in a guanidine hydrochloride solution; HRP, horseradish peroxidase; Mrcp,
Megabalanus rosa cement protein; nMrcp-20k, protein extracted from the secondary cement in pure water; rMrcp-20k, recombinant form of
Mrcp-20k expressed in Escherichia coli.
6436 FEBS Journal 274 (2007) 6436–6446 ª2007 The Authors Journal compilation ª2007 FEBS

crustacean’s own calcareous base and the foreign sub-
stratum, which can be a metal oxide, synthetic polymer,
or the calcareous shell of another animal, in water. Cal-
cific material is necessarily the most frequently encoun-
tered target for attachment by the barnacle cement.
So far, four cement proteins have been identified,
with different characteristics [2]. No homologous pro-
teins have been found in other organisms. Among the
four cement proteins produced by the barnacle,
cp-100k and cp-52k are the two major components in
terms of amount, and are characterized by their insolu-
ble nature [3]. These two components are considered
to constitute the bulk region of the cement. A reducing
treatment with guanidine hydrochloride was necessary
to render the bulk proteins soluble. cp-68k is also a
major protein, whose amino acid composition is heav-
ily biased towards four amino acids, i.e. Ser, Thr, Ala,
and Gly, although the specific function of this protein
in underwater attachment is not known at present [3].
cp-20k is a minor cement protein in terms of its
amount, and is not post-translationally modified. The
amino acid composition of cp-20k is characterized by
the unusual abundance of Cys (17%) and charged
amino acids (Asp, 11.5%; Glu, 10.4%; His, 10.4%) [4].
Although the high abundance of the Cys residue in the
protein has suggested a possible contribution to inter-
molecular crosslinking or coupling [5], our previous
study has indicated that this is not the case, at least
with respect to the latter speculation [4].
Underwater attachment is a multifunctional process,
which is different from that of an artificial adhesive in
air, and is thus an unachievable technique at present.
The process [6] involves such subfunctions as prevent-
ing random aggregation during transport via the
cement duct, displacing sufficient seawater to prime
and spread on the surface without being dispersed in
the water, coupling strongly with a variety of material
surfaces, and self-assembly to join the calcareous base
and the substratum. After the process, it is then neces-
sary to cure the cement so that the holdfast remains
stiff and tough, and to protect it from microbial degra-
dation. The insoluble nature of the complex and the
limitations of microanalytical methods for studying
each function, however, have hindered elucidation of
the specific function of each cement protein [3].
There are two types of sample for studies on barnacle
cement: primary cement and secondary cement [1,3].
Primary cement is a natural adhesive of a few microme-
ters in thickness between the base and foreign substra-
tum, whereas secondary cement is secreted when the
animal is free from a substratum. Both forms of cement
are similar in their whole amino acid composition [7],
and appear to contain the same protein components as
determined by peptide mapping with cyanogen bromide
treatment [3]. Reattachment of the barnacle to a new
substratum by secondary cement has also been reported
[1,8], although the adhesive strength was weaker than
that of primary cement. The primary cement seemed to
be denser and more rigid than the secondary cement.
Although these studies indicated that the primary and
secondary cements have the same protein composition,
it is not clear whether the protein–protein interactions
and the topology in the two complexes are the same.
Megabalanus rosa (Mr)cp-20k in the secondary
cement was chemically characterized in a previous
study [4]. However, neither the nature of Mrcp-20k in
the primary cement nor the specific function of this
protein in underwater attachment has been unraveled.
The present study was performed to characterize the
nature of the protein in the primary cement. Thereaf-
ter, we expressed the recombinant form of the protein
in bacteria in a soluble form under physiological con-
ditions, and confirmed that the recombinant protein
has almost the same structure as that of the native bar-
nacle protein. We subsequently showed that the recom-
binant protein has a specific affinity for calcite surfaces
in water. This is the first report to identify a biotic
underwater adhesive protein as a specific adsorbent to
calcite, by directly measuring the adsorbing activity of
the protein prepared under physiological conditions.
Results
Confirmation of Mrcp-20k in natural barnacle
cement
Mrcp-20k was extracted only from guanidine hydro-
chloride-soluble fraction 1 (GSF1) of the primary
cement, but not from GSF2, which is the guanidine
hydrochloride-soluble fraction after reducing treatment
(Fig. 1A). This result is consistent with what is found
in the secondary cement [4]. Mrcp-20k in GSF1 of the
primary cement only gave a band with a monomeric
molecular mass on SDS ⁄PAGE without the reducing
treatment (Fig. 1A); this is also consistent with what is
found for the secondary cement [4]. This indicates that
Mrcp-20k is not covalently crosslinked in the natural
cement. Mrcp-20k was not detected in the peripheral
shell (Fig. 1B), indicating that Mrcp-20k is not a
protein related to calcification of the shell.
Preparation of the recombinant form of Mrcp-20k
in bacteria
The recombinant form of Mrcp-20k in Escherichia coli,
rMrcp-20k, was purified in solution under physiologi-
Y. Mori et al.Calcite-coupling protein in underwater adhesive
FEBS Journal 274 (2007) 6436–6446 ª2007 The Authors Journal compilation ª2007 FEBS 6437
cal conditions (Fig. 2A). The elution profiles from
both RP-HPLC and ion exchange HPLC were identi-
cal to those of native Mrcp-20k in the secondary
cement extracted in pure water, nMrcp-20k (supple-
mentary Fig. S1A,B). Owing to the vector construc-
tion, rMrcp-20k was designed to have an additional
tripeptide, Ala-Met-Ala, attached to the N-terminus.
The N-terminal sequence and molecular mass of the
recombinant protein were determined to be AMAHE-
EDGV and 20 629 Da, respectively, which agree well
with the deduced sequence and mass (20 629.3 Da).
This molecular mass corresponds to the form of the
protein in which all Cys residues form disulfide bonds.
Alkylation treatment of rMrcp-20k resulted in a same
mass, suggesting that no free SH groups are present in
rMrcp-20k. The presence of all Cys residues in the
intramolecular disulfide form in the recombinant pro-
tein is the same as what is found for the protein in the
secondary cement [4]. SDS ⁄PAGE analysis showed
that rMrcp-20k without a reduction treatment had a
slightly lower mobility than that with the reduction
treatment (Fig. 2B); this resembles the behavior of the
native Mrcp-20k protein in the secondary cement. The
CD spectrum of rMrcp-20k in a 10 mmsodium phos-
phate buffer (pH 6.8) was also identical to that of
nMrcp-20k; both showed the presence of a mixture of
b-turn and random coil structures [9,10]. These spectra
were remarkably different from that observed after a
reducing treatment, probably due to denaturation of
the protein (Fig. 3).
Adsorption of rMrcp-20k to underwater material
surfaces
The adsorption of rMrcp-20k to several underwater
material surfaces was investigated, and the findings
are summarized in Fig. 4. The protein was adsorbed
to calcite in artificial seawater (ASW), whereas it was
not adsorbed to glass, gold, polystyrene, or benzo-
guanamine-formaldehyde resin, which is a positively
charged synthetic polymer. The protein was also
adsorbed to a limited extent to metal oxides such as
zinc oxide and magnetite. The amount adsorbed to
calcite in pure water was almost the same as that in
ASW.
A
B
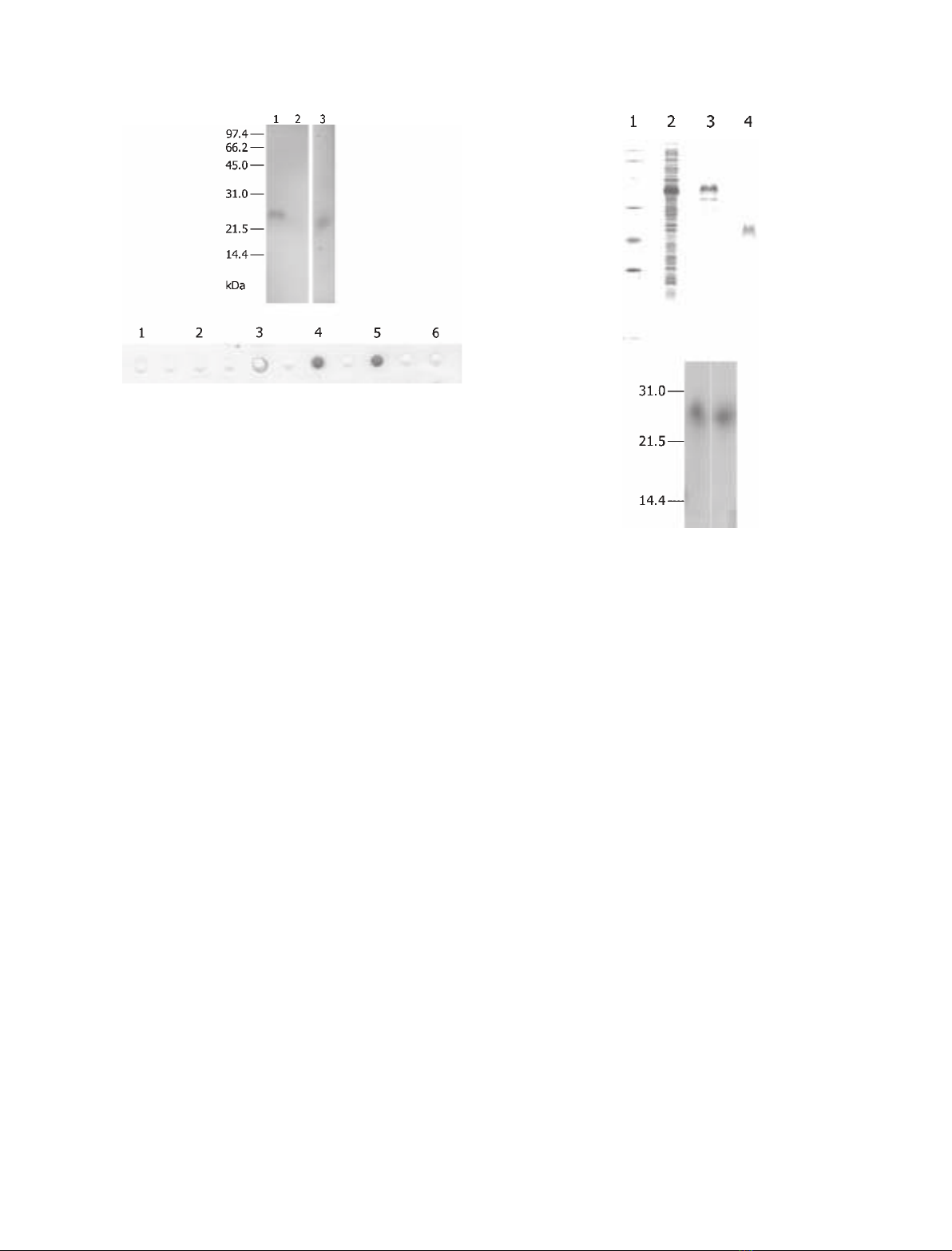
Fig. 2. Purification of rMrcp-20k. (A) Samples were separated by
using the 16.5% T Tris ⁄Tricine buffer system of SDS ⁄PAGE [30].
Lane 2: crude extract of bacterial cells. Lane 3: rMrcp-20k fused
with a tag in the vector construct. Lane 4: rMrcp-20k. Lane 1, low
molecular mass markers (Bio-Rad; aldolase, 45.0 kDa; carbonic
anhydrase, 31.0 kDa; soybean trypsin inhibitor, 21.5 kDa; lysozyme,
14.4 kDa). (B) SDS ⁄PAGE of rMrcp-20k with (left) and without
(right) pretreatment with the reducing agent 2-mercaptoethanol.
A
B
Fig. 1. Characterization of Mrcp-20k in the primary cement. (A)
Western blotting of fractions rendered soluble from the primary
cement by using the antibody to Mrcp-20k. Lane 1: GSF1 with
reduction pretreatment in SDS ⁄PAGE. Lane 2: GSF2 with reduction
pretreatment. Lane 3: GSF1 without reduction pretreatment. Num-
bers on the left-hand side indicate molecular masses (kDa). (B)
Detection of Mrcp-20k in the peripheral shell of the barnacle by
using the antibody to Mrcp-20k. Two grams each (dry weight) of
the peripheral shell and calcareous base were decalcified and
subjected to dot-blotting. Lane 1: 2% acetic acid solution–soluble
fraction of the peripheral shell. Lane 2: GSF1 and GSF2 of the
peripheral shell. Lane 3: 2% acetic acid solution–soluble fraction of
the base. Lane 4: GSF1 and GSF2 of the base. Lane 5: rMrcp-20k
as positive control (1 lg). Lane 6: trypsin inhibitor from soybean as
negative control (1 lg; Wako Pure Chemical Industries).
Calcite-coupling protein in underwater adhesive Y. Mori et al.
6438 FEBS Journal 274 (2007) 6436–6446 ª2007 The Authors Journal compilation ª2007 FEBS
The relationship between the concentration of the
protein at the calcite surface and its solution concen-
tration is described by the adsorption isotherm. The
linearized forms of the isotherm for the adsorption to
calcite were C
eq
⁄Q¼0.3168 ·10
)3
+ 4.199C
eq
[corre-
lation coefficient (r
2
) of 0.97] in ASW and
C
eq
⁄Q¼1.7168 ·10
)3
+ 3.782C
eq
(r
2
of 0.98) in the
dilute buffer [C
eq
, equilibrium protein concentration;
Q, amount of absorbed protein (lmol) per m
2
of the
surface] (Fig. 5). The slope and intercept of the result-
ing lines enabled us to estimate the adsorption affinity
(K) and the maximum number of adsorption sites (N)
to be K¼1.33 ·10
7
m
)1
and N¼2.38 ·10
)7
molÆm
)2
in ASW, and K¼2.20 ·10
6
m
)1
and N¼
2.64 ·10
)7
molÆm
)2
in the dilute buffer solution. The
isotherms for adsorption to zinc oxide and magnetite
were not linear (r
2
of 0.75 and 0.58, respectively), so
that the adsorption to these surfaces seemed not to be
of the typical Langmuir type (supplementary Fig. S2).
The adsorption of rMrcp-20k to the barnacle shell
was visualized using the antibody to rMrcp-20k with
the secondary antibody conjugated by fluorochrome
(Fig. 6 and supplementary Fig. S3). A 10 min incuba-
tion with rMrcp-20k in ASW gave rise to fluorescence
emission at the barnacle shell, demonstrating the
Wavelength (nm)
[θ] (deg cm-2 dmol-1)
200
-30
-20
-10
0
10
[θ] (deg cm-2 dmol-1)
-30
-20
-10
0
10
[θ] (deg cm-2 dmol-1)
-30
-20
-10
0
10
A
B
C
250 300 320
Wavelength (nm)
200 250 300 320
Wavelength (nm)
200 250 300 320
Fig. 3. Comparison of the CD spectra of rMrcp-20k and nMrcp-
20k. The spectra are shown of (A) rMrcp-20k, (B) nMrcp-20k and
(C) rMrcp-20k with the reducing pretreatment.
A
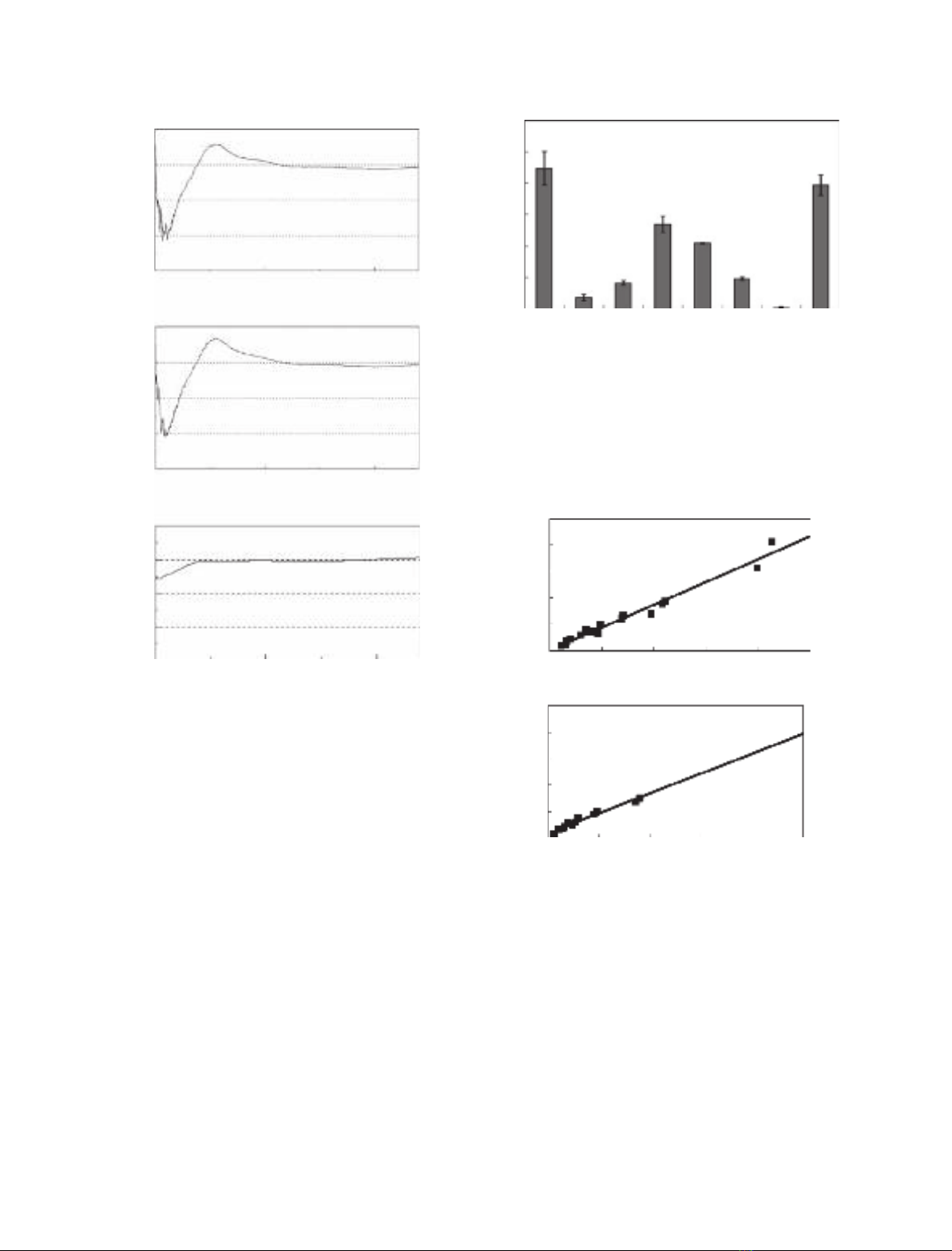
amount of adsorbed protein (ng/cm2)
0
50
100
150
200
250
300
BCDEFGH
Fig. 4. Adsorption of rMrcp-20k to various solid surfaces. The
adsorption of rMrcp-20k to the particles of several materials in
10 min at 25 C was evaluated by measuring the decrease in pro-
tein amount remaining in the solution. Adsorption to (A) calcite in
ASW, (B) glass in ASW, (C) benzoguanamine–formaldehyde resin
in ASW, (D) zinc oxide in ASW, (E) magnetite in ASW, (F) gold in
ASW, (G) polystyrene in ASW, and (H) calcite in pure water. Error
bars indicate the standard deviation.
Ceq (µmol/mL)
Ceq/Q (m2/mL)
-5.2E-18
0
0.01
0.02
0.03
0.04
0.05
Ceq/Q (m2/mL)
0
0.01
0.02
0.03
0.04
0.05
B
A
0.002 0.004 0.006 0.008 0.01
Ceq (µmol/mL)
-2.08E-1 0.002 0.004 0.006 0.008 0.01
Fig. 5. Linearized adsorption isotherm for adsorption of rMrcp-20k
to calcite. (A) Isotherm in ASW. (B) Isotherm in 2.14 mMsodium
carbonate (pH 8.2).
Y. Mori et al.Calcite-coupling protein in underwater adhesive
FEBS Journal 274 (2007) 6436–6446 ª2007 The Authors Journal compilation ª2007 FEBS 6439
successful adsorption of the protein to the calcareous
shell of the barnacle.
The distribution of the molecular size of
rMrcp-20k
The distribution of the molecular size of the recombi-
nant protein was evaluated by analytical ultracentrifu-
gation (Table 1).
Sedimentation velocity analyses indicated that the
protein exists as a single component in 100 mmto
500 mmNaCl solution. The sedimentation coefficient
of the component was estimated to be s2.5.
The sedimentation equilibrium analyses gave nearly
20 kDa as the molecular mass in 100 mmto 1 mNaCl
solution, which is consistent with monomeric molecu-
lar mass of the protein. Therefore, the s2.5 species
found by sedimentation velocity corresponds to the
monomeric form of the protein.
The possible change of intramolecular disulfide
bonds to intermolecular ones after a longer period of
incubation in ASW was evaluated by SDS ⁄PAGE
analysis (Fig. 7). The molecular masses were mono-
meric for proteins in both the suspension and the lay-
ers adsorbed to calcite, thus confirming that there had
been no change of intramolecular disulfide bonds to
the intermolecular type in the protein.
Isolation of the homologous gene from Balanus
albicostatus
A PCR investigation of a homologous gene in three
barnacle species was attempted with several degener-
ated oligonucleotide primers based on the primary
structure of Mrcp-20k. All PCR trials with primers
designed from the primary structure of Mrcp-20k failed
to amplify homologous DNA, except for 3¢-RACE with
cDNA of Balanus albicostatus. The sequence of homo-
logous cDNA in B. albicostatus determined in this
study was 700 bp, and the coding region was deter-
mined to encode 125 amino acids (supplementary
Fig. S4). The first 20 amino acids are considered to
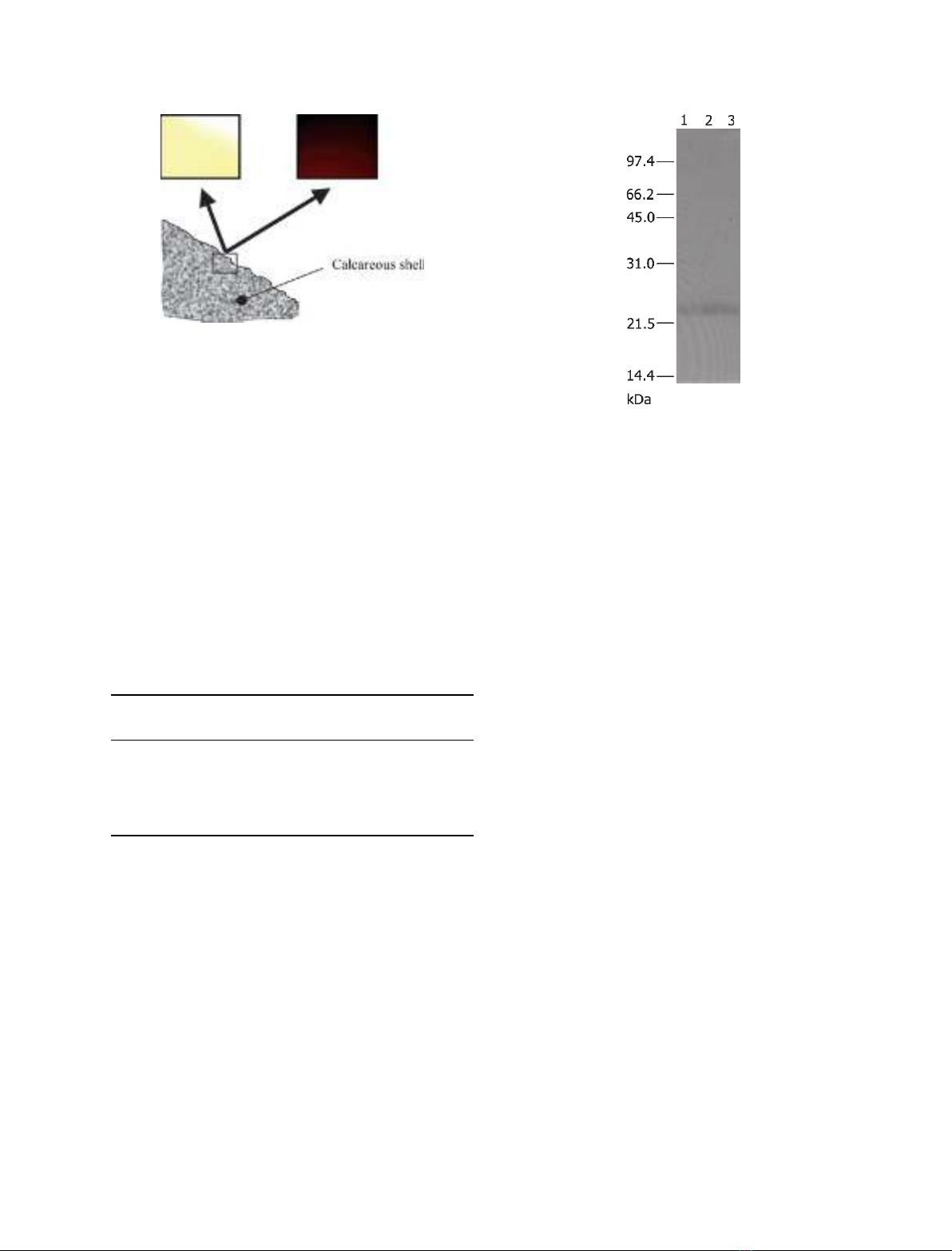
Fig. 6. Demonstration of the adsorption of Mrcp-20k to the barna-
cle peripheral shell. The protein adsorbed to the shell was treated
with the antibody, and visualized with the secondary antibody
linked to fluorochrome Cy3 (GE Healthcare Bio-Science). Images
under visible light (left) and those under reflected fluorescence
(right) are shown. The image pair was captured from the same
angle of the object. In the images under visible light, yellow areas
correspond to the shell, and white areas are transparent without
any object. Shell was incubated with rMrcp-20k, washed, and trea-
ted with the antibody to Mrcp-20k. No fluorescence was observed
in the control experiment (supplementary Fig. S3).
Table 1. The distribution of the molecular size of rMrcp-20k evalu-
ated by analytical ultracentrifugation. The sedimentation coefficients
and molecular masses of rMrcp-20k in several solvents were evalu-
ated by sedimentation velocity and sedimentation equilibrium,
respectively. Sedimentation coefficients were evaluated by sedi-
mentation velocity analyses and standardized with the SEDNTERP pro-
gram [29]. Molecular masses were determined by sedimentation
equilibrium analyses.
NaCl concentration (M)
s
20, W
(S)
Molecular
mass (kDa)
0.1 2.6 19.6
0.3 2.5 18.9
0.4 2.5 –
0.5 2.4 –
1.0 – 21.1
Fig. 7. Rearrangement of disulfide bonds in rMrcp-20k during long-
term incubation. The molecular masses of rMrcp-20k after several
treatments for 1 week at 25 C were estimated by western blotting
with the antibody to Mrcp-20k antibody. rMrcp-20k was incubated
in ASW adjusted to pH 8.0 without calcite particles (lane 1), in a
dilute buffer adjusted to pH 8.0 without calcite particles (lane 2), or
in ASW with calcite particles (lane 3).
Calcite-coupling protein in underwater adhesive Y. Mori et al.
6440 FEBS Journal 274 (2007) 6436–6446 ª2007 The Authors Journal compilation ª2007 FEBS
















![Báo cáo seminar chuyên ngành Công nghệ hóa học và thực phẩm [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250711/hienkelvinzoi@gmail.com/135x160/47051752458701.jpg)









