
Characterization of chitinase-like proteins (Cg-Clp1 and
Cg-Clp2) involved in immune defence of the mollusc
Crassostrea gigas
Fabien Badariotti, Christophe Lelong, Marie-Pierre Dubos and Pascal Favrel
Universite
´de Caen Basse-Normandie, IBFA, UMR M100 IFREMER, Physiologie et Ecophysiologie des Mollusques Marins, Laboratoire de
Biologie et Biotechnologies Marines, Caen, France
Glycoside hydrolase family 18 (GH18) is a phylogenet-
ically conserved group of proteins present in eukaryo-
tes, prokaryotes and viruses. The GH18 family is
characterized by a Glyco_18 domain adopting an
(a⁄b)
8
triose phosphate isomerase-barrel structure that
consists of a specific arrangement of eight parallel
b-strands, forming the barrel core, surrounded by eight
a-helices [1]. This family classification, based only on
similarities in amino acid sequences, groups together
chitinases and proteins devoid of catalytic activity due
to the substitution of a critical amino acid in the cata-
lytic centre. This latter singular class of proteins, called
chitinase-like proteins (CLPs), has been identified only
recently in plants [2], mammals [3], insects [4] and mol-
luscs [5]. CLPs have been implicated in many biologi-
cal processes, such as control of nodulation [2] and
growth ⁄differentiation balance during development in
plants [6]. Insect CLPs such as imaginal disc growth
factors represent the first proliferating polypeptides
reported from invertebrates [7]. These mitogenic
growth factors cooperate with insulin to stimulate pro-
liferation, polarization and mobility of imaginal disc
cells in vitro. Imaginal disc growth factors may also
constitute morphogenetic factors controlling embryonic
and larval development, and could stimulate the cell
growth required for wound healing [8,9]. In mammals,
CLPs such as YM1 ⁄2 and YKL-40 (40 kDa mamma-
lian protein with the N-terminus YKL) [also known as
human cartilage glycoprotein-39 (HC-gp39) in humans]
are considered to be cytokines [10,11] involved in tis-
sue remodelling during pathological conditions [12,13].
Recently, the first lophotrochozoan CLP was identified
Keywords
chitinase-like protein; Crassostrea gigas;
immunity; lectin; mollusk
Correspondence
P. Favrel, Universite
´de Caen Basse-
Normandie, IBFA, UMR M100 IFREMER,
Physiologie et Ecophysiologie des
Mollusques Marins, 14032 Caen cedex,
France
Fax: +33 231565346
Tel: +33 231565361
E-mail: pascal.favrel@unicaen.fr
(Received 22 February 2007, revised
10 May 2007, accepted 23 May 2007)
doi:10.1111/j.1742-4658.2007.05898.x
Chitinase-like proteins have been identified in insects and mammals as non-
enzymatic members of the glycoside hydrolase family 18. Recently, the first
molluscan chitinase-like protein, named Crassostrea gigas (Cg)-Clp1, was
shown to control the proliferation and synthesis of extracellular matrix
components of mammalian chondrocytes. However, the precise physiologi-
cal roles of Cg-Clp1 in oysters remain unknown. Here, we report the clo-
ning and the characterization of a new chitinase-like protein (Cg-Clp2)
from the oyster Crassostrea gigas. Gene expression profiles monitored by
quantitative RT-PCR in adult tissues and through development support its
involvement in tissue growth and remodelling. Both Cg-Clp1- and Cg-
Clp2-encoding genes were transcriptionally stimulated in haemocytes in
response to bacterial lipopolysaccharide challenge, strongly suggesting that
these two close paralogous genes play a role in oyster immunity.
Abbreviations
Cg-Clp1 ⁄2, Crassostrea gigas chitinase-like protein 1 ⁄2; CLP, chitinase-like protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase;
GH, glycoside hydrolase; HC-gp39, human cartilage glycoprotein-39 (also called YKL-40); LPS, lipopolysaccharide; YKL-40, 40 kDa
mammalian protein with the N-terminus YKL.
3646 FEBS Journal 274 (2007) 3646–3654 ª2007 The Authors Journal compilation ª2007 FEBS

from the oyster Crassostrea gigas [5]. Interestingly, this
protein, named C. gigas chitinase-like protein 1 (Cg-
Clp1) was found to be involved in the control of
growth and remodelling processes in a manner similar
to its YKL-40 mammalian counterpart. These findings
argue for an early evolutionary origin and a high con-
servation of this class of proteins at both the structural
and functional levels. Given the multiplicity of CLPs
in humans and insects [14], we hypothesized that
homologues of the previously characterized Cg-Clp1
remain to be found in C. gigas.
In this article, we report the characterization of a
new CLP, named Cg-Clp2, from the oyster C. gigas.
The tissue distribution and temporal pattern of expres-
sion of the gene encoding Cg-Clp2 during oyster devel-
opment were established by real-time PCR and in situ
hybridization. In addition, the involvement of both
Cg-Clp2 and the previously identified Cg-Clp1 in oys-
ter immune defence was established.
Results
Isolation and sequence analysis of Cg-Clp2
full-length cDNA
RT-PCR with degenerate primers whose design was
based on the conserved amino acid sequences of the
catalytic domain of members of the GH18 family
resulted in the amplification of an expected 147 bp
sequence. Cloning and sequencing of this fragment
revealed an ORF showing amino acid sequence simi-
larity to members of the GH18 family. Subsequently,
specific primers deduced from this 147 bp sequence
were used to perform 5¢- and 3¢-RACE-PCR to obtain
the full-length cDNA. This experimental strategy has
been applied successfully in former studies, leading to
the identification of the two first C. gigas members of
the GH18 family, Cg-Clp1 (AJ971241) [5] and the chi-
tinase Cg-Chit (AJ971238) [15]. The complete 1697 bp
cDNA (AJ971235) revealed an ORF of 1425 bp, start-
ing with an ATG at position 117 and ending with a
TAA at position 1542. This ORF encodes a protein
named C. gigas chitinase-like 2, composed of 475
amino acids with a putative N-terminal 19 amino acid
signal peptide (Fig. 1). Cg-Clp2 contains one potential
recognition site for N-linked oligosaccharide [16] and
two potential recognition sites for O-linked oligosac-
charide [17] (http://www.cbs.dtu.dk/services) (Fig. 1).
Cg-Clp2 sequence identity with other proteins
Optimal alignment of Cg-Clp2 with Cg-Clp1 and other
GH18 family members revealed regions of significant
identity, especially in the Glyco_18 domain. The glu-
tamate residue known to be critical for chitinase activ-
ity [18] is substituted by a glutamine, suggesting that
this protein lacks chitinolytic activity, as was shown
previously for recombinant Cg-Clp1 [5] and other
CLPs [19]. Following the Glyco_18 domain, Cg-Clp2
displays an additional 90 amino acid C-terminal
sequence of unknown function (Fig. 1). Hence, the
overall structure of Cg-Clp2 is similar to that of
Cg-Clp1.
Expression of Cg-Clp2 transcripts during
development and in adult tissues
To gain insights into possible physiological functions
of Cg-Clp2, determination of its tissue distribution and
temporal pattern of expression during development
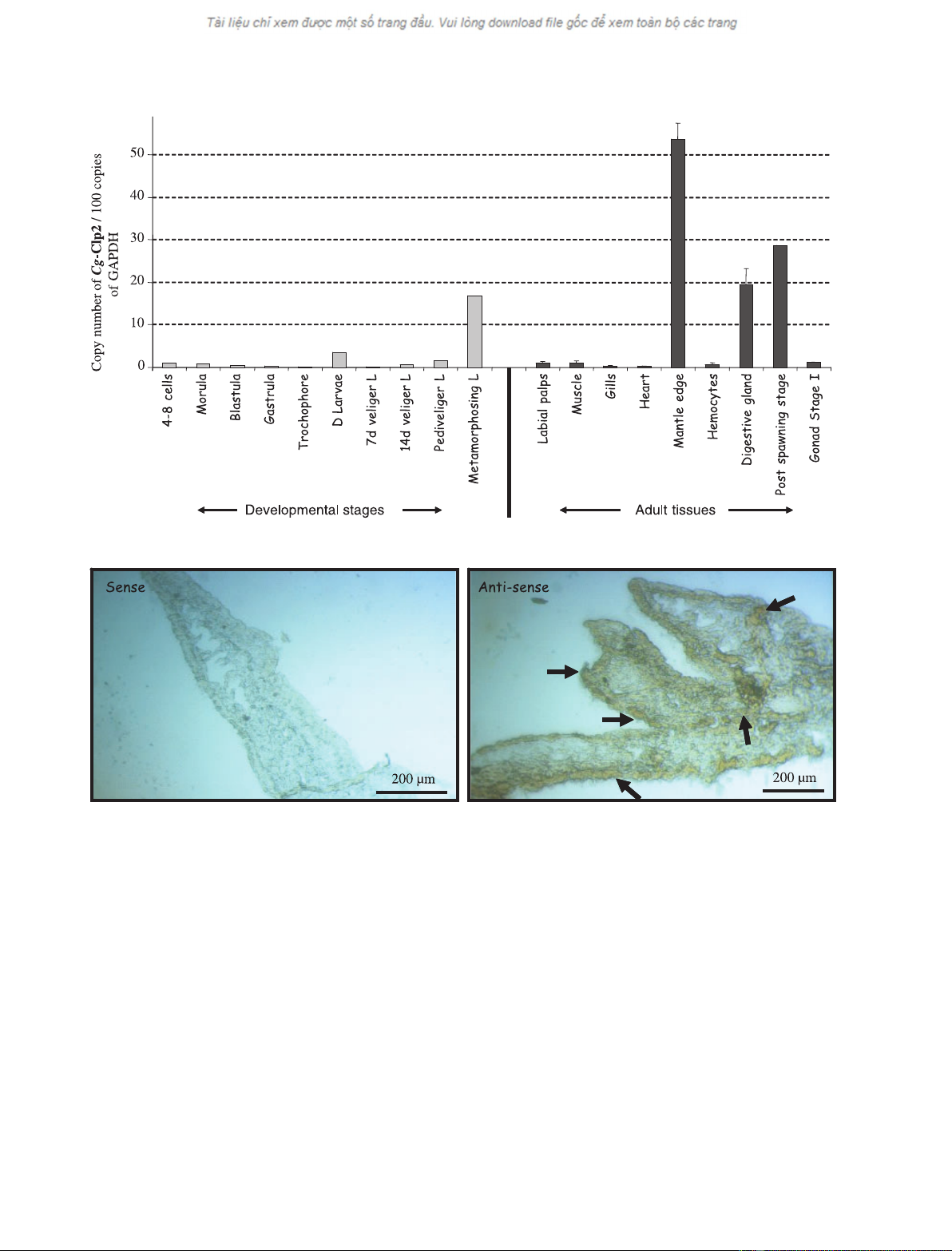
was performed by real time RT-PCR (Fig. 2A). Cg-
Clp2 transcripts were mainly expressed during larval
metamorphosis, in the mantle edge and the digestive
gland. During the reproductive cycle, expression was
high in gonads during the postspawning period but
not in stage I, when gonial multiplication starts [20].
To investigate which types of cell were responsible for
Cg-Clp2 expression in the mantle edge, in situ hybrid-
ization experiments were performed (Fig. 2B). Tran-
scripts were detected in both epithelial and conjunctive
cells of the mantle.
Cg-Clp1 and Cg-Clp2 mRNA levels are increased
in haemocytes after bacterial LPS challenges
As the two mammalian CLPs, YM1 and YKL-40,
were recently categorized as immune cytokines [10,11],
the possible involvement of Cg-Clp1 and Cg-Clp2 in
oyster immunity was investigated. Gene expression was
analysed by real-time RT-PCR in haemocytes at differ-
ent times after injection of bacterial lipopolysaccharide
(LPS) into the posterior adductor muscle and after an
in vitro LPS challenge.
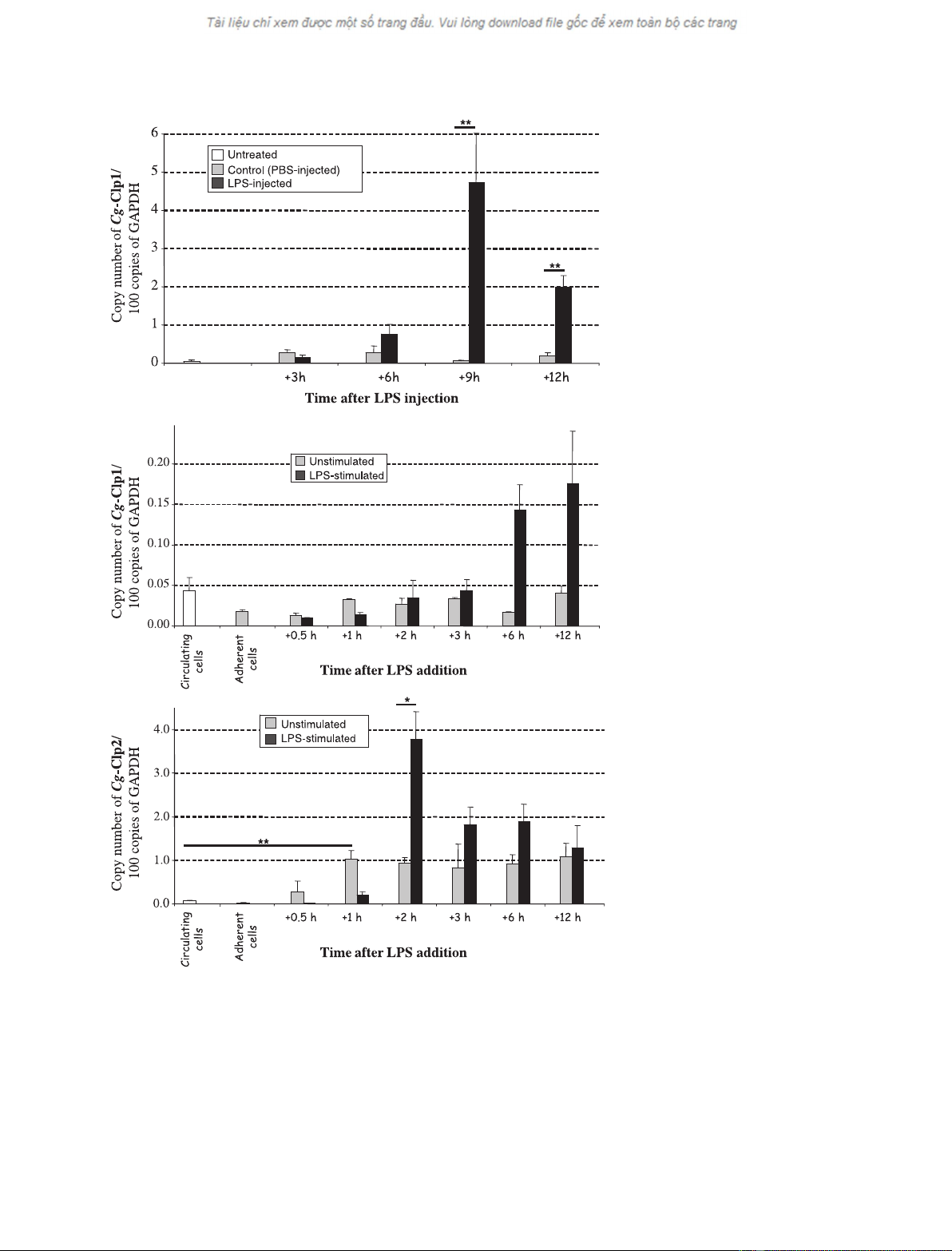
A marked increase in Cg-Clp1 expression was
observed in vivo 9 h and 12 h after LPS injection
(Fig. 3A) relative to the respective controls. Cg-Clp1
was also transcriptionally stimulated in vitro, 6 h and
12 h after LPS addition, in comparison to unstimulat-
ed control haemocytes (Fig. 3B). However, this upreg-
ulation was substantially lower than that observed for
in vivo challenge. In contrast, Cg-Clp2 expression was
not affected by in vivo LPS challenge (data not shown)
but, as compared to unstimulated haemocytes, was
stimulated in vitro 2 h after LPS addition (Fig. 3C).
Surprisingly, the Cg-Clp2 expression level was also
significantly enhanced in adherent nonstimulated
F. Badariotti et al. Oyster chitinase-like proteins
FEBS Journal 274 (2007) 3646–3654 ª2007 The Authors Journal compilation ª2007 FEBS 3647
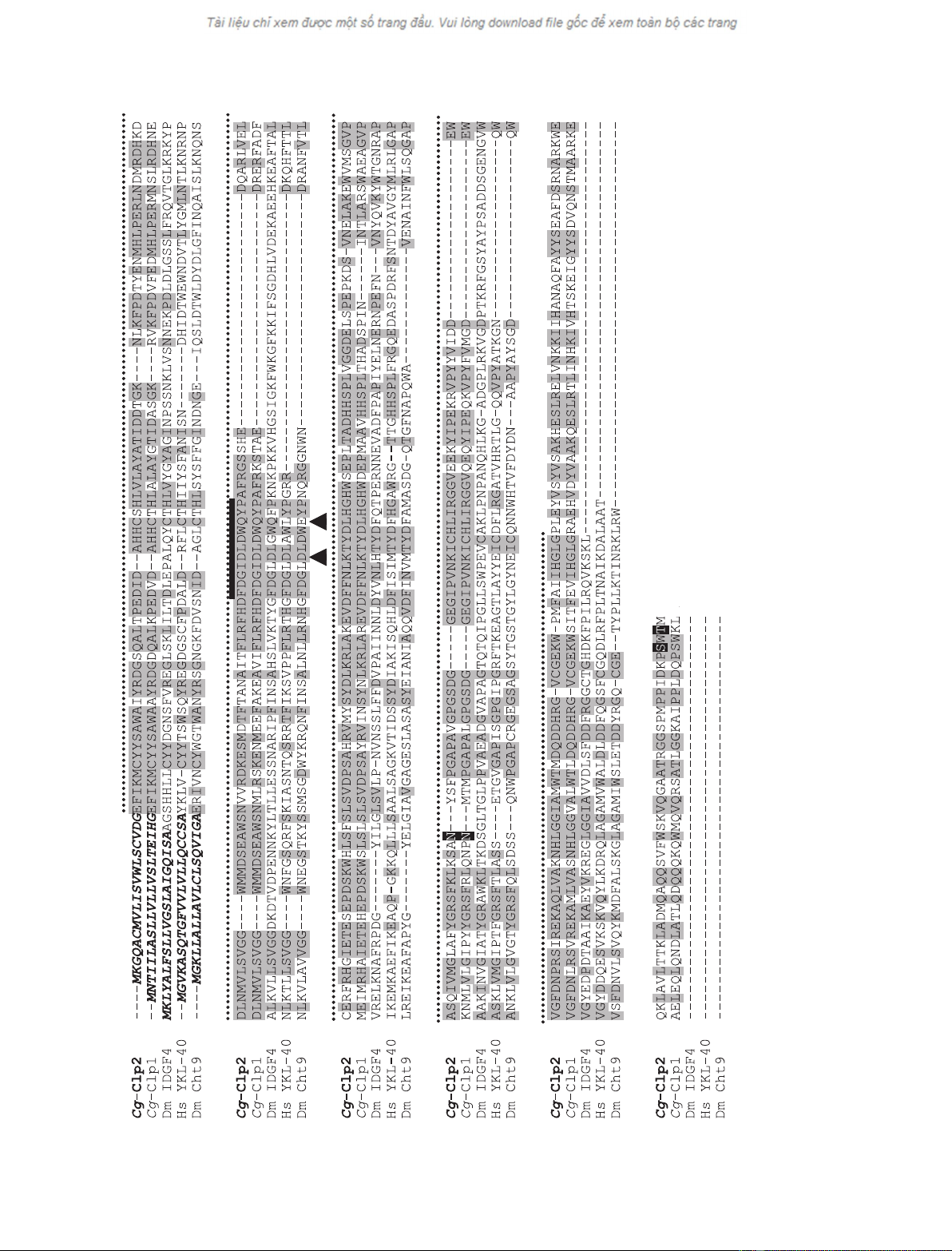
Fig. 1. Multiple sequence alignment of Cg-Clp2 with members of the GH18 family. (A) The predicted amino acid sequence of Cg-Clp2 is aligned with the amino acid sequence of three
CLPs from the oyster C. gigas (Cg-Clp1), Drosophila melanogaster (IDGF4) and Homo sapiens (YKL-40), and with the sequence of the Drosophila chitinase Cht9. Conserved residues (iden-
tical to Cg-Clp2) are shaded in dark grey. Potential sites for N-glycosylation (NXT ⁄S) and for O-glycosylation (S or T) are shaded in black. Amino acids of the predicted signal peptide are
shown in bold italic letters. Dashes indicate gaps in the amino acid sequence when compared with other sequences. The GH18 conserved sequence motif including the catalytic residues
is marked with a thick black line above the sequence alignment. Arrowheads indicate the positions of residues (D and E) required for catalytic activity in bacterial chitinases [18]. The black
dotted line delimits the Glyco_18 domain. The species abbreviations used are: Dm, Drosophila melanogaster; and Hs, Homo sapiens. GeneBank accession numbers: Cg-Clp1, AJ971241;
Dm IDGF4, NP511101; Hs YKL-40, NP001267; Dm Cht9, NP611543.
Oyster chitinase-like proteins F. Badariotti et al.
3648 FEBS Journal 274 (2007) 3646–3654 ª2007 The Authors Journal compilation ª2007 FEBS
haemocytes as compared to freshly harvested circula-
ting cells.
Discussion
In the present study, we identified a second oyster
CLP named Cg-Clp2. Comparative sequence analyses
with other GH18 family members show that Cg-Clp2
displays the same protein organization as the previ-
ously identified Cg-Clp1, with a Glyco_18 domain (in
a catalytically inactive form [5]) followed by an addi-
tional C-terminal sequence of about 90 amino acids of
unknown function. The high degree of identity of the
Cg-Clp1 and Cg-Clp2 Glyco_18 domains (84% iden-
tity) argues for a conservation of the tertiary structure
and associated biochemical properties (such as chitin
binding). Evidence for a high level of conservation of
the tertiary structure of CLPs during evolution is also
supported by the observation that both Cg-Clp1 and
its closest mammalian homologue YKL-40 present
A
B
Fig. 2. Expression of Cg-Clp2 mRNAs in adult tissues and during development measured by real-time quantitative RT-PCR. (A) Each value is
the mean + SE of three pools of four animals (tissues) or the mean of one pool of embryos or larva from one spawn. Expression levels are
related to 100 copies of GAPDH. (B) Localization of Cg-Clp2 mRNA expression in the mantle edge investigated by in situ hybridization.
Arrows indicate hybridization signals.
F. Badariotti et al. Oyster chitinase-like proteins
FEBS Journal 274 (2007) 3646–3654 ª2007 The Authors Journal compilation ª2007 FEBS 3649
similar biological activities on mammalian chondro-
cytes [5]. As YKL-40 is only composed of the sole
Glyco_18 domain, the C-terminal tail of C. gigas CLPs
may not noticeably contribute to the structure and the
function of these proteins. Interestingly, Cg-Clp1 and
Cg-Clp2 C-terminal regions share relatively low levels
of sequence identity (46%), probably as the result of a
lower pressure of selection during evolution. Neverthe-
less, these discrepancies may also account for slightly
distinct biochemical properties.
Analysis of mRNA distribution during development
and in adult tissues shows that Cg-Clp2 is expressed
A
B
C
Fig. 3. Real time quantitative RT-PCR
analysis of Cg-Clp1 and Cg-Clp2 mRNA
expression in haemocytes following bacter-
ial LPS challenges. In vivo experiment: time-
dependent effect of LPS (100 lg) injection
on Cg-Clp1 expression (A). Results are
means + SE of at least three oysters.
In vitro experiment: time-dependent effect
of LPS addition (final concentration
13 lgÆmL
)1
) to cell culture medium on
Cg-Clp1 (B) and Cg-Clp2 (C) expression.
Results are means + SE of three wells.
Statistical analysis of the results was per-
formed with Student’s t-test (*P< 0.05;
**P< 0.02).
Oyster chitinase-like proteins F. Badariotti et al.
3650 FEBS Journal 274 (2007) 3646–3654 ª2007 The Authors Journal compilation ª2007 FEBS


























