Two conserved domains in regulatory B subunits mediate
binding to the A subunit of protein phosphatase 2A
Xinghai Li
1
and David M. Virshup
1,2
1
Department of Oncological Sciences, Center for Children, Huntsman Cancer Institute, and
2
Department of Pediatrics,
University of Utah, Salt Lake City, UT, USA
Protein phosphatase 2A (PP2A) is an abundant heterotri-
meric serine/threonine phosphatase containing highly con-
served structural (A) and catalytic (C) subunits. Its diverse
functions in the cell are determined by its association with a
highly variable regulatory and targeting B subunit. At least
three distinct gene families encoding B subunits are known:
B/B55/CDC55, B¢/B56/RTS1 and B¢¢/PR72/130. No
homology has been identi®ed among the B families, and little
is known about how these B subunits interact with the PP2A
A and C subunits. In vitro expression of a series of B56a
fragments identi®ed two distinct domains that bound inde-
pendently to the A subunit. Sequence alignment of these A
subunit binding domains (ASBD) identi®ed conserved resi-
dues in B/B55 and PR72 family members. The alignment
successfully predicted domains in B55 and PR72 subunits
that similarly bound to the PP2A A subunit. These results
suggest that these B subunits share a common core structure
and mode of interaction with the PP2A holoenzyme.
Keywords: phosphoprotein phosphatase; PP2A; subunit
interactions; phosphorylation.
Protein phosphatase 2A (PP2A) is an abundant cellular
serine/threonine-speci®c phosphatase that regulates a sig-
ni®cant array of cellular events. The PP2A holoenzyme is a
heterotrimer, containing a 65-kDa regulatory A subunit
(A/PR65), a 36-kDa catalytic C subunit, and one of a
variety of regulatory B subunits. These diverse B subunits in
the PP2A heterotrimer allow the phosphatase to localize to
distinct regions of the cell and to dephosphorylate speci®c
substrates, thereby allowing PP2A to regulate diverse
processes in the cell such as DNA replication, Wnt
signaling, apoptosis, and cytoskeletal function (reviewed in
[1,2]). The importance of B subunits in cellular regulation is
illustrated by the effect of mutations that alter B subunit
function. Over-expression of B56 blocks Wnt signaling in
Xenopus embryos [3±5], mutations in a Drosophilia B/B55
subunit leads to imaginal disc duplication and defects in
mitosis [6,7], transposon insertions in B56cenhance the
metastatic ability of mouse melanoma cell lines [8], muta-
tions in the A subunit that alter B subunit binding are found
in lung, breast, colorectal and skin cancers [9,10], and
decreases in A subunit expression are seen in neuronal
tumors [11]. Despite the signi®cant role the B subunits play
in cellular homeostasis, little is known about how they
physically interact with the PP2A holoenzyme to target the
phosphatase to its substrates.
The PP2A A subunit serves as a scaffold for assembly of
the B and C subunits. It is composed of 15 imperfect HEAT
repeats, each of 39 amino acids, which form a hook-shaped
molecule [12]. The repeats consist of two ahelices connected
by an intrarepeat loop, and mutations in distinct loops alter
the binding of the B and C subunits [13]. The B subunits
bind to repeats 1±10 of the A subunit, whereas the C subunit
binds to repeats 11±15. Interactions between the B and C
subunits are also important for heterotrimer formation, as
loss of C subunit binding sites prevents B subunit binding
[14,15], and modi®cation of the C-terminus of the C subunit
regulates B subunit binding [16±18].
To date, at least three families of PP2A B subunits have
been identi®ed in eukaryotes. They are designated B (PR55,
B55, CDC55), B¢(PR61, B56, RTS1), and B¢¢ (PR72/130).
Each B subunit family is encoded by multiple genes, with
multiple splice variants, generating an extraordinary diver-
sity of these regulatory subunits [1,2]. Although the three
families of B subunits do not share apparent sequence
similarities between the families, they do have signi®cant
sequence homology within each family. For example, within
the B56 family, each isoform shares a common core region
of 241 amino acids with 71±88% identity by protein
sequence, while both the N- and C-termini are signi®cantly
more divergent [19±21]. The conserved core region has been
proposed to interact with the AC heterodimer, while the
nonconserved N- and C-ends may perform different
functions, such as regulation of substrate speci®city and
subcellular targeting [20,22]. Two additional classes of
polypeptides also interact with the AC core of PP2A. Both
the small and middle T antigens encoded by polyomavirus
and SV40, and the calmodulin-binding proteins striatin and
SG2NA [23], bind to the AC core of PP2A. However, unlike
the B subunits, T antigens and striatin do not require
interaction with, nor methylation, of the PP2A C subunit
[17].
Little is known about the molecular basis for the
interaction of the B subunits with the AC heterodimer.
None of the B subunits have been mapped to de®ne the
Correspondence to D. M. Virshup, Huntsman Cancer Institute,
University of Utah, Salt Lake City, UT 84112. Fax: + 801 587 9415,
Tel.: + 801 585 3408, david.virshup@hci.utah.edu
Abbreviations: PP2A, protein phosphatase 2A; ASBD, A subunit
binding domain; GST-A, glutathione S-transferase A subunit; NP-40,
nonidet p40; CMV, cytomegalovirus.
(Received 19 September 2001, revised 8 November 2001, accepted 16
November 2001)
Eur. J. Biochem. 269, 546±552 (2002) ÓFEBS 2002
A subunit binding domains. In this study, we used the B56a
isoform as a model regulatory protein to identify structural
elements involved in the interaction with PP2A. We
identi®ed two distinct domains within the B56acore region
that are each suf®cient for interaction with the A subunit.
Sequence alignment analyses demonstrated that these two
distinct regions are signi®cantly conserved among the three
eukaryotic B subunit families. The predicted A subunit
binding domains in B/B55 and B¢¢/PR72 were also able to
interact with the PP2A A subunit. The presence of a
conserved motif in the highly divergent B subunits suggests
a common ancestry, structure, and mode of A subunit
interaction for these important regulatory proteins.
EXPERIMENTAL PROCEDURES
Synthesis of [
35
S]protein
[
35
S]Methionine-labeled B subunits and their fragments, and
SV40 small t antigen and its mutant were generated by
coupled in vitro transcription and translation in rabbit
reticulocyte lysates (TNT, Promega) using PCR-generated
templates. All N-terminal PCR primers incorporated a T3
or T7 promoter sequence. Ampli®ed PCR products were
puri®ed using a PCR puri®cation kit (Qiagen) and
200±400 ng of puri®ed DNA was added to 50 lLof
reticulocyte lysate in the presence of [
35
S]methionine. The
reaction was incubated at 30 °C for 2 h. In several cases,
additional lower molecular mass bands were seen which are
likely to be due to either premature termination or partial
proteolysis of the [
35
S]methionine-labeled proteins.
Preparation of glutathione
S
-transferase (GST)
and GST-A fusion proteins
The GST-A subunit of PP2A (GST-A) construct was a
generous gift from M. Mumby (UT Southwestern, Dallas,
TX, USA) [24]. Puri®cation of GST-A and GST proteins
from Escherichia coli was performed as described previously
[24]. The puri®ed proteins were thoroughly dialyzed against
buffer A (50 m
M
Tris/HCl pH 7.5, 20 m
M
NaCl, 2 m
M
EDTA, 1 m
M
dithiothreitol, containing 3 lgámL
)1
pepsta-
tin and leupeptin, 2 m
M
benzamidine, and 1 m
M
phen-
ylmethanesulfonyl ¯uoride). The resultant protein
preparation was stored at )70 °C in buffer A containing
50% glycerol until use.
GST precipitation assay
The binding reactions contained 10 lLof[
35
S]methionine-
labeled polypeptides from programmed reticulocyte lysates,
2lg of GST or GST-A and buffer A in a ®nal volume of
50 lL. After incubation for 2 h at ambient temperature (or
4hat30°C, where indicated), the reaction was diluted to
500 lL with buffer B [buffer A containing 0.1% nonidet
p40 (NP-40) and 0.25% BSA] and 20 lLofapre-
washed 1 : 1 slurry of glutathione±Sepharose (Amersham
Pharmacia) was added. Incubation continued for 2 h at
4°C. The beads were then washed four times with 1 mL of
buffer B, or RIPA buffer (50 m
M
Tris, pH 7.5, 150 m
M
NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) where
indicated, for 10 min each wash. Bound proteins were then
eluted by incubating the beads with 20 lLof10m
M
reduced glutathione in buffer A on ice for 30 min The
eluted polypeptides were analyzed by either conventional
SDS/PAGE or on tricine/glycine gels for small molecular
mass peptides [25] and imaged using a Molecular Dynamics
PhosphorImager.
RESULTS AND DISCUSSION
Identi®cation of two A subunit-binding domains in B56a
To determine the minimal region of B56 that interacted with
the PP2A subunit, we utilized an in vitro binding assay using
GST-A subunit and reticulocyte lysate-synthesized B frag-
ments [10,21]. To optimize conditions for the assay, full-
length B56awas ®rst tested for binding to GST-A. B56a
full-length protein bound well to GST-A, but not to GST
alone (Fig. 1A). To further con®rm the speci®c binding,
SV40 small t antigen and a truncation mutant were used as a
binding control. Consistent with previous reports, GST-A
speci®cally bound to wild-type small t, but not to a mutant
small t antigen lacking the A subunit binding site (m#3,
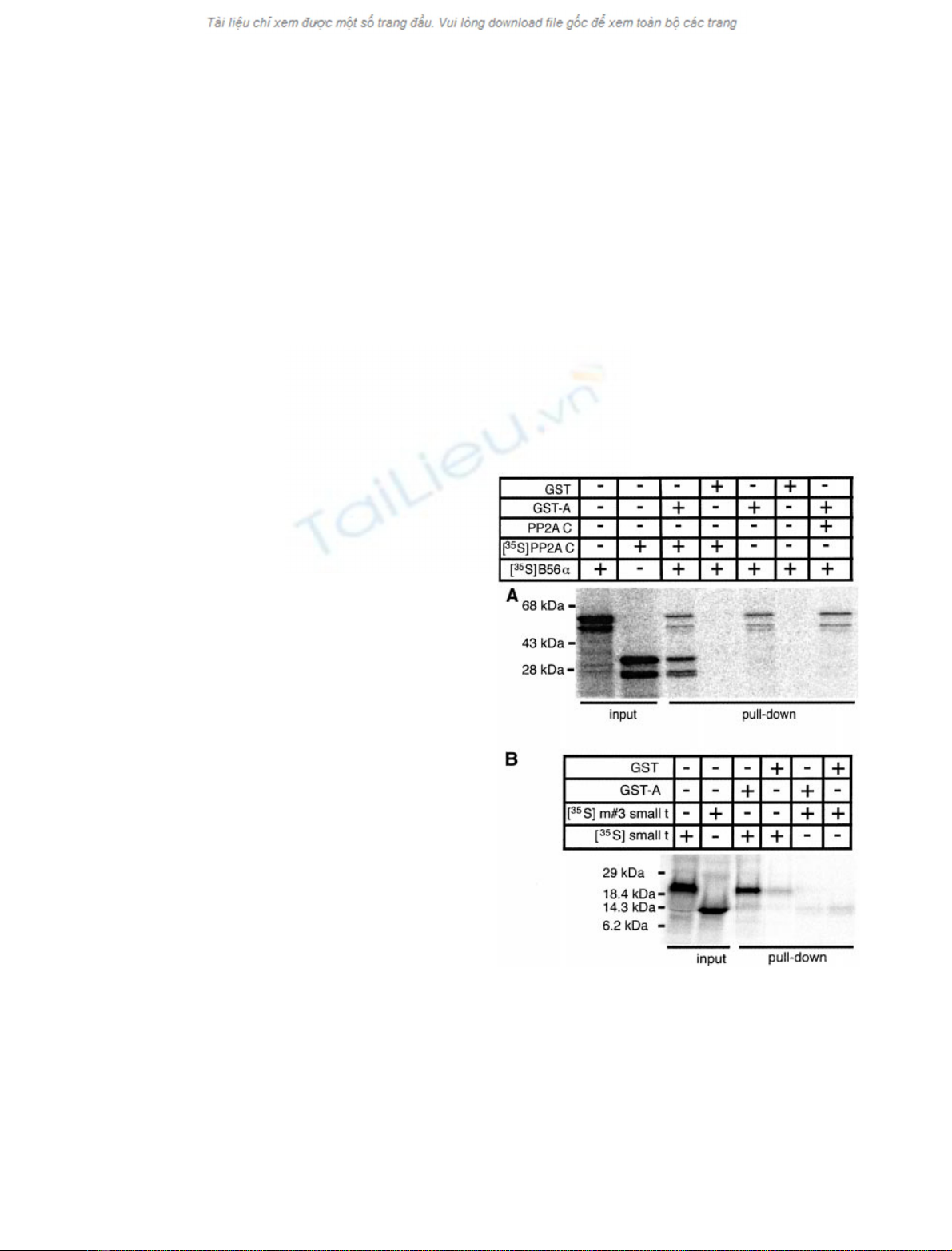
Fig. 1. Binding of B56ato the A subunit of PP2A. [
35
S]Methionine-
labeled proteins generated in vitro were incubated with GST or GST-A
for 2 h at ambient temperature, and precipitated with glutathione±
Sepharose beads. The bound proteins were eluted with the reduced
glutathione and analyzed by SDS/PAGE followed by PhosphorImager
analysis. (A) Added C subunit does not enhance the GST-A:B56a
interaction. Binding of B56awild type protein to PP2A A was assessed
in the presence or absence of 1 lg of puri®ed PP2A C and/or 10 lLof
35
S-labeled PP2A C synthesized in vitro. (B) GST-A bound speci®cally
to the full-length SV40 small t, but not to the m#3 mutant small
t (1±110 fragment).
ÓFEBS 2002 Conserved PP2A A subunit binding domains (Eur. J. Biochem. 269) 547

small t 1±110 fragment, Fig. 1B) [24,26]. Also consistent
with previous reports, we saw no enhancement of B56a
binding when the reactions were supplemented with puri®ed
C subunit or [
35
S]methionine-labeled C subunit synthesized
in the reticulocyte lysate (Fig. 1A), suggesting the C subunit
present in the reticulocyte lysate may contribute to the
formation of heterotrimers [27].
To map the region(s) of B56aresponsible for binding to
the A subunit, multiple B56 fragments were generated by
PCR followed by in vitro transcription and translation. The
ability of the fragments to bind to GST-A was assessed as
described above and the results shown in Fig. 2. Two
distinct domains that interacted with GST-A but not the
GST control were identi®ed. Generally less than 10% of
input B56awas recovered from the glutathione±Sepharose
beads when GST-A subunit was included. This low recovery
may be due to a high level of nonspeci®c adsorption of the
B56apolypeptides to the beads, and suboptimal binding in
the absence of cotranslation of the A and C subunits. The
smallest N-terminal fragment of B56athat interacted with
GST-A encompasses residues 200±303 (Fig. 2). A second
domain extending from amino acids 325±383 was capable of
independently binding to GST-A (Fig. 2). These regions
were named A subunit binding domains (ASBD) 1 and 2.
Given that the two distinct regions can bind to the
structural A subunit, an effort was undertaken to express
these domains in vivo. We reasoned that over-expression of
an A subunit binding domain at high levels might displace
endogenous B subunits, thereby blocking speci®c interac-
tions with substrates and leading to alterations in speci®c
signaling pathways. A series of epitope-tagged B56afrag-
ments (amino acids 1±142, 142±303, 200±383, 303±383, and
383±486) were expressed in human embryonic kidney
(HEK293) cells using a cytomegalovirus (CMV)-promoter
driven construct. Unfortunately, only the 1±142 fragment
was highly expressed by immunoblot analysis, while the
142±303 fragment was barely expressed in comparison with
expression of the full-length protein (1±486). Expression of
other B56afragments was not detectable (data not shown).
Similar results were obtained with two additional expression
vectors. In addition, fusion of green ¯uorescent protein to
either end of a polypeptide containing B56aamino acids
180±383 did not result in detectable protein. Considering
that these fragments can be well expressed in reticulocyte
lysates, it seems likely that the failure to detect the expressed
fragments in cultured cells is due to enhanced degradation
by intracellular proteases. One possibility is that these B56a
fragments have substantially lower af®nity for the PP2A AC
heterodimer than does full-length B56a. As B56 subunits
over-expressed in vivo are detected only in PP2A heterotri-
mers [20], B subunits and their fragments unable to be
stabilized by PP2A binding in vivo may be inherently
unstable and rapidly lost.
Identi®cation of two conserved regions present
in all three families of B subunits
Although no apparent sequence homology has been
discovered among B subunits of the three families identi®ed
thus far, all B subunits do bind to overlapping N-terminal
regions of PP2A A (intraloop repeats 1±10) [13,27]. These
data suggest the possibility that B subunits contain common
structural elements that are responsible for the PP2A A
binding. To test whether these two PP2A A binding
domains identi®ed in B56aare conserved among different
B subunits, the
CLUSTALW
multiple sequence alignment
program (available at http://workbench.sdsc.edu) was used
to align a diverse collection of B subunits (either functionally
identi®ed or characterized by sequence homology from
various species) against these two domains. While full-
length B56 failed to produce a signi®cant alignment with
other B subunits, homology with B/B55 and PR72 family
members was found when only the B56 binding domains
were used in the alignment (Fig. 3). For ASBD 1, the region
of homology (amino acids 188±292 of hsB56a) substantially
overlaps the experimentally deduced A subunit binding
domain (amino acids 200±303), while for ASBD 2, the
overlap is even tighter (homology, 329±386; binding 325±
383). Conserved hydrophobic, charged, and polar residues
are distributed along the length of the two domains. The
two domains are separated by a less-conserved region of
between 20 and 41 amino acids. A conserved amino-acid
pro®le (Fig. 3) was generated by visual inspection of the two
aligned sequences, and used to search the nonredundant
protein database at the Swiss Institute for Experimental
Cancer Research web site (http://www.isrec.isb-sib.ch).
Each pro®le identi®ed over 95% of the approximately 105
B/B55/CDC55, B¢/B56/RTS1, and B¢¢/PR72 related seq-
uences contained in the database. Neither pro®le identi®ed
any novel types of B subunits, strongly suggesting no
additional conventional B subunit families exist, at least in
the nonredundant protein database. Neither pro®le identi-
®ed irrelevant proteins. The pro®les did not match SV40
and polyomavirus t antigens nor members of the striatin/
SG2NA families, implying these PP2A-interacting proteins
have a distinct ancestry and mechanism of interaction.
Notably, the pro®les identi®ed B, B56, and PR72-type B
subunits in organisms as diverse as Neurospora crassa,
Candida tropicalis,Dictyostelium discoideum,Medicago
varia (alfalfa), Arabidopsis thaliana,Oryza sativa (rice),
Caenorhabditis elegans,Drosophila melanogaster,Xenopus
laevis, and mammals. Combining the ASBD 1 and 2 pro®les
with a variable linker between them also identi®ed over 90%
of the B subunits in the database. Similar results were
obtained when a
PROSITE
pro®le, generated from the
multiple sequence alignment data using the
MOTIF
program
at http://www.motif.genome.ad.jp was used to search the
Swiss-Prot protein database. We conclude that these pro®les
accurately re¯ect conserved amino acids in the PP2A B
subunit families.
Fig. 2. Binding of full-length and truncated B56ato the A subunit of
PP2A. [
35
S]Methionine-labeled reticulocyte lysate-synthesized B56a
and fragments were mixed with GST-A or GST for 2 h at the ambient
temperature as described, and the resultant complexes were precipi-
tated with glutathione±Sepharose beads. After washing, bound com-
plexes were eluted with reduced glutathione and analyzed by
SDS/PAGE and PhosphorImager. (A) Schematic summary of the
binding properties of the B56afragments. The empty bar represents
full-length B56aor its fragments, and the gray boxes represent the
deduced A subunit-binding domains. (B) Representative autoradio-
graphs from the binding assays. The left panel shows 5 lL of input
reticulocyte lysate, and the right panel demonstrates which B56a
fragments precipitated with GST-A and GST beads. Each experiment
was repeated at least three times with similar results.
548 X. Li and D. M. Virshup (Eur. J. Biochem. 269)ÓFEBS 2002



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





