
A peptide derived from cyclin-dependent kinase activator (p35)
specifically inhibits Cdk5 activity and phosphorylation of tau protein
in transfected cells
Ya-li Zheng, Bing-Sheng Li, Niranjana D. Amin, Wayne Albers and Harish C. Pant
Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, USA
Cyclin-dependent kinase-5 (Cdk5) is a serine/threonine
kinase activated by its neuron-specific activator, p35, or its
truncated form, p25. It has been proposed that the deregu-
lation of Cdk5 activity by association with p25 in human
brain tissue disrupts the neuronal cytoskeleton and may be
involved in neurodegenerative diseases such as Alzheimer’s
disease. In this study, we demonstrate that a short peptide
(amino acid residues 154–279; Cdk5 inhibitory peptide;
CIP), derived from p35, specifically inhibits Cdk5 activity
in vitro and in HEK293 cells cotransfected with the
peptide and Cdk5/p25, but had no effect on endogenous
cdc2 kinase activity. Moreover, we demonstrate that the
phosphorylation of tau in HEK293 cells, cotransfected with
Cdk5/p25 and CIP, is effectively reduced. These results
suggest that CIP specifically inhibits both Cdk5/p25 complex
activity and the tau hyperphosphorylation induced by Cdk5/
p25. The elucidation of the molecular basis of p25 activation
and CIP inhibition of Cdk5 activity may provide insight into
mechanisms underlying the pathology of Alzheimer’s dis-
ease and contribute to therapeutic strategies.
Keywords: Cdk5, p35, Cdk5 inhibitory peptide (CIP), Tau
phosphorylation, Alzheimer’s disease.
Cdk5 is a serine/threonine kinase with close homology to
the mitotic Cdks [1,2]. It plays a critical role in brain
development and neuronal migration [3–5]. In contrast to
other members of the Cdk family, Cdk5 is activated by
binding the neuron-specific noncyclin molecules, p35 or p39
[6–9]. Mice lacking p35 are viable and fertile but show
lamination defects in the cerebral cortex and mild disruption
in the hippocampus and cerebellum [10], whereas mice
deficient in Cdk5 die perinatally and show severe and
widespread defects in neuronal migration [3–5,11]. p35/
Cdk5 kinase activity promotes neurite growth and phos-
phorylates a wide variety of substrates [12]. Deregulation of
Cdk5 activity by proteolytic conversion of p35 to p25 has
been implicated in neurodegenerative diseases [13,14].
Computer modeling and mutagenesis studies have pre-
dicted that p35 adopts a cyclin-like tertiary structure
[15–17]. Although, to produce full activity, in addition to
cyclin binding most members of the Cdk family require
phosphorylation of an intramolecular domain called the
T-loop by another kinase [18]. Cdk5 differs in that full
activity can be achieved by binding to p35 in the absence of
T-loop phosphorylation [17,19].
The p35 activation domain was mapped to the region of
amino acid residues 150–291 [16,17]. More recently Amin
et al. found that residues 138–291 constitute the smallest
fragment (p16) of p35 that fully activates Cdk5 [20]
(Fig 1A). That study found that further truncation of p16,
removing either the N-terminal 11 residues (part of the
p35 aNT helix, Fig. 1A) or the C-terminal four residues of
p16 (the p35 a7 helix, Fig. 1A), produces peptides that bind
to Cdk5 with moderate affinity and do not activate it in vitro,
but instead competitively inhibit. Remarkably, the peptide
that remains after both C- and N-terminal truncations (p35
residues 154–279) has a much higher affinity for Cdk5. This
Cdk5 inhibitory peptide (CIP) markedly inhibits the activity
of Cdk5 in vitro [20]. The high affinity of CIP suggested that
it might act as a specific Cdk5 inhibitor in a cellular
environment as well. We explored this possibility by
examining the specificity of its inhibition in HEK293 cells
transfected with Cdk5/p25. We find that CIP specifically
inhibits the activity of Cdk5/p25 but does not affect the
activity of cdc2 kinase in transfected HEK293 cells. We also
observed that CIP reduces the phosphorylation of tau in
HEK293 cells cotransfected with tau, CIP and Cdk5/p25.
These results indicate that transfection of CIP efficiently and
specifically inhibits Cdk5/p25 complex activity and, in p25-
transfected cells, reduces tau phosphorylation. Finally, we
discuss the molecular basis of CIP inhibition of Cdk5/p25
activity in relation to the recently published Cdk5/p25
crystal structure [21].
MATERIALS AND METHODS
Materials
p35 (N-20), p35 (C-19) polyclonal antibody, Cdk5 (C-8)
polyclonal antibody, Cdk5 (J-3) monoclonal antibody, cdc2
P34 (H-297) polyclonal antibody, and cdc2 p34 (17)
monoclonal antibody were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Anti-tau (AT8) Ig
Correspondence to H. C. Pant, Laboratory of Neurochemistry,
NINDS, NIH, Bldg. 36, Rm 4D04, 9000 Rockville Pike,
Bethesda, MD 20892-4130, USA.
Fax: + 1 301 496 1339, Tel.: + 1 301 402 2124,
E-mail: panth@ninds.nih.gov
Abbreviations: Cdk5, cyclin-dependent kinase-5; CIP, Cdk5 inhibitory
peptide; HEK293, human embryonic kidney 293.
(Received 2 May 2002, revised 2 July 2002, accepted 23 July 2002)
Eur. J. Biochem. 269, 4427–4434 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03133.x
was obtained from Innogenetics (Gent, Belgium), PS202
polyclonal antibody (phosphorylated tau epitopes at
Ser202) and TAU-5 monoclonal antibody (reacts with the
nonphosphorylated as well as the phosphorylated forms of
tau) were purchased from Biosource International,
Inc (Camarillo, CA, USA). Other antibodies include
anti-Xpress–HRP and anti-Xpress–FITC (which detect
fusion proteins containing the eight amino acid Xpress
epitope: Asp-Leu-Tyr-Asp-Asp-Asp-Asp-Lys; Invitrogen,
Carlsbad, CA, USA) and anti-T7 Tag monoclonal antibody
(Novagen, San Diego, CA, USA). A pcDNA/Amp euk-
aryotic expression vector and LipofectAMINE Reagent
were purchased from Invitrogen (Carlsbad, CA). pGEM-T
vector was obtained from Promega Corporation (Madison,
WI, USA). The Cdk5 inhibitor, roscovitine, was obtained
from Biomol Research Laboratories, Inc. (Plymouth
Meeting, PA).
Plasmids and constructs
Construction of CMV expression vectors for Cdk5, p35,
and p25 were made according to a procedure described by
Nikolic et al. [22]. A 126-residue peptide (CIP) correspond-
ing to peptide fragment Cys154 to Pro279 of p35 was
made by PCR using a forward primer (5¢-
TGCCTGGGTGAGTTTCTC-3¢) and a reverse primer
(5¢-TGGGTCGGCATTTATCTG-3¢) derived from p35
(Fig. 1A). A CMV-tau fragment (amino acids 181–242)
was generated by PCR from a rat brain cDNA library.
The primer was designed according to the rat tau
sequence, as follows: a forward primer (5¢-ACACCACC
CAGCTCTGGT-3¢) and a reverse primer (5¢-GCG
GCTCTTGGCGGAAGA-3¢). The PCR amplified frag-
ments were gel purified with a GenecleanII kit (Bio101, Inc.
from BCH Medical Supplies Co.). After cutting with Not1
and EcoRI, the fragments were inserted into Not1/EcoRI
site of a linearized CMV (pcDNAC3) vector. The constructs
of CIP and tau peptides were verified by sequencing.
Cell culture and transfection
Human embryonic kidney (HEK293) cells were obtained
from the American Type Culture Collection, cultured in
Dulbecco’s modified Eagle’s medium with 10% fetal
bovine serum, supplemented with 100 UÆmL
)1
penicillin
and 100 lgÆmL
)1
streptomycin at 37 C in a humidified
atmosphere of 5% CO
2
. The cells were transiently
transfected using LipofectAMINE (Life Technologies)
according to the manufacturer’s instructions. The above
described constructs of CIP, p25, p35, wild-type Cdk5 and
tau were transfected independently or cotransfected.
Twenty-four hours post-transfection, the cells were starved
in the presence of 0.2% fetal bovine serum overnight (to
reduce any background stimulation by serum factors). The
cells were fixed for immunocytochemistry analysis, or
lysed with lysis buffer for immunoprecipitation and
Western blot analysis.
Western blot analysis
Cells were harvested by scraping from dishes and lysed in
ice-cold lysis buffer (20 m
M
Tris, pH 7.5, 150 m
M
NaCl,
1m
M
EDTA, 1 m
M
EGTA, 1% Triton X-100, 0.1% SDS,
2.5 m
M
sodium pyrophosphate, 1 m
M
2-glycerol phos-
phate, and 1 m
M
Na
3
VO
4
, supplemented with a mixture of
protease inhibitors and 1 m
M
phenylmethanesulfonyl fluo-
ride) by passing through a 21 gauge needle several times and
incubation for 30 min on ice. After centrifugation for
20 min at 13 000 gat 4 C, the protein concentrations of
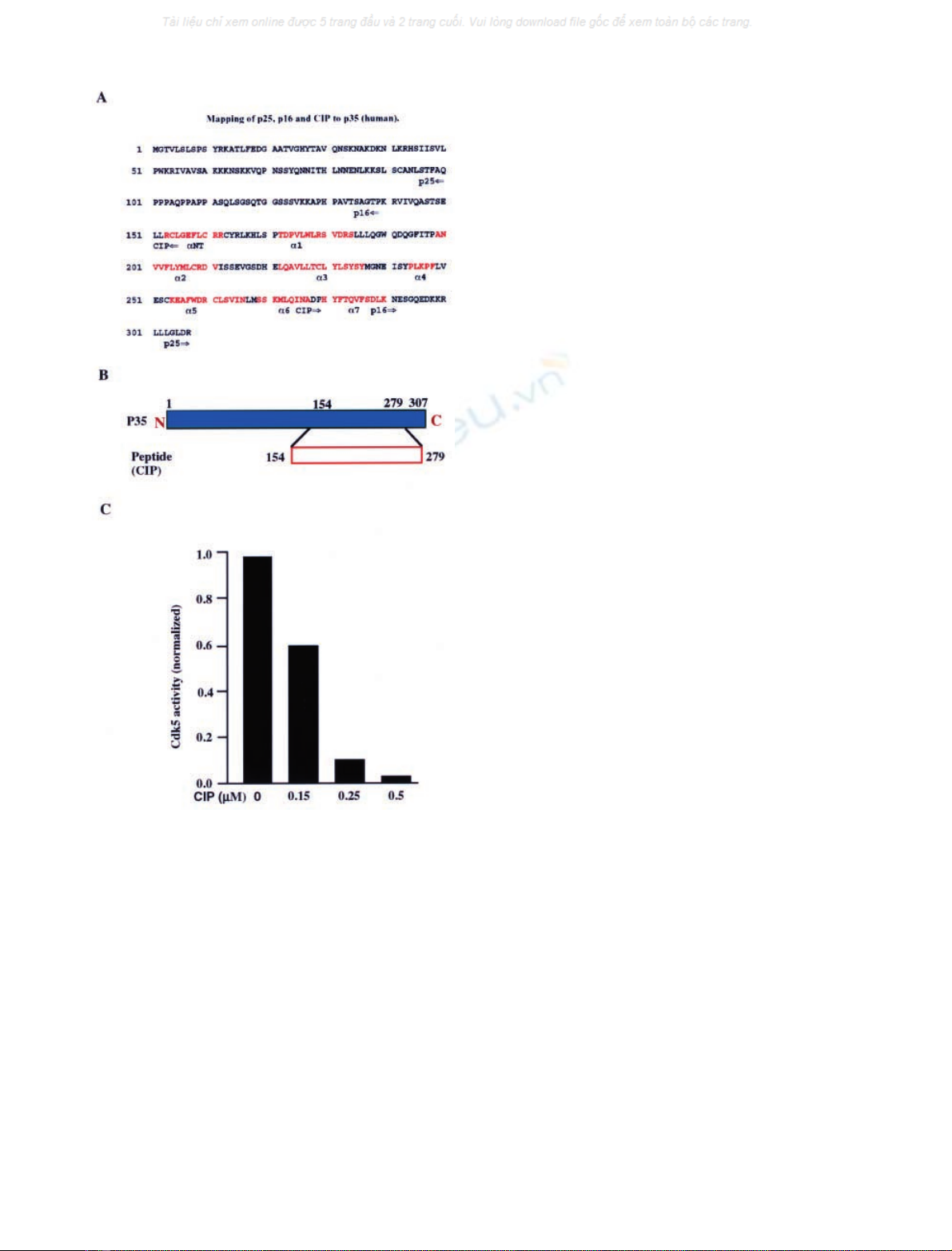
Fig. 1. Identification of the Cdk5/p25 inhibitory peptide (CIP) derived
from p35. (A) Mapping of p25, p16 and CIP to p35 (human sequence).
Red segments are the alpha helices in p25 as determined by Tarricone
et al. [21]. The sequences comprising p25, p16 and CIP are indicated by
the labelled arrows. (B) A combination of N-terminal and C-terminal
truncations of p35 produces a nonactivating fragment (154–279, CIP)
that inhibits Cdk5 activity and binds with high affinity. (C) Inhibition
of Cdk5 activity by CIP. Cdk5 kinase activity was determined by
preincubating various amount of CIP with Cdk5/p25 for 2 h at 30 C
followed by incubation in the kinase reaction for an additional hour in
the presence of [c-
32
P]ATP and histone H1 as described in the mate-
rials and methods section.
4428 Y.-l. Zheng et al. (Eur. J. Biochem. 269)FEBS 2002

the supernatants were determined using BCA protein
reagent. An equal amount of total protein (20 lgof
protein per lane) was resolved on a 4–20% SDS-polyacryl-
amide gel and blotted onto a poly(vinylidene difluoride)
membrane. This membrane was blocked by incubating in
blocking buffer containing 20 m
M
Tris/HCI (pH 7.4),
150 m
M
NaCI, and 0.1% (v/v) Tween 20 (Tris/NaCl/
Tween) plus 5% dry milk (w/v) for 1 h at room temperature.
This was followed by incubation overnight at 4 Cin
primary antibodies: anti-Cdk5 (C-8, 1 : 200), p35 (N-20 and
C-19, 1 : 200), anti-Xpress–HRP (1 : 3000), anti-cdc2 P34
(H-297 and 17, 1 : 200), anti-PS202, and TAU-5 mAb
(1 : 1000 and 1 : 500, respectively) diluted in blocking
buffer. The membranes were then washed in Tris/NaCl/
Tween (4 ·5 min). This was followed by incubation in
secondary antibody (goat- anti-mouse or goat anti-(rabbit
IgG H + L)–HRP conjugate at a dilution of 1 : 3000) for 2
h at room temperature and washing four times in Tris/
NaCl/Tween. Western blots were analyzed using the
Amersham Enhanced Chemiluminescence (ECL) kit fol-
lowing the manufacturer’s instructions.
Immunoprecipitation and kinase assays
Cells were lysed in ice-cold lysis buffer without SDS,
described as above, and immunoprecipitated with anti-
Cdk5 (C-8), anti-cdc2 P34 (17) or anti-Xpress. The
immunoprecipitates were washed twice with lysis buffer
and twice with kinase buffer. Kinase activity assays were
performed as described previously [23]. In brief, a total
volume of 50 lL of kinase assay mixture was used,
containing 50 m
M
Tris/HCl (pH 7.4) with 1 m
M
EGTA,
1m
M
dithiothreitol, 5 m
M
MgCI
2
,0.5m
M
micro-
cystinLR, 10 lg of histone H1, and 10 lL of cdk5 or
Xpress immunoprecipitates. The phosphorylation reaction
was initiated by the addition of 0.1 m
M
[c-
32
P]ATP and
incubated at 30 C for 30 min. The reaction was termin-
ated by spotting 25 lL of the reaction mixture on P81
phosphocellulose pads that were washed five times in
75 m
M
phosphoric acid followed by rinsing with 95%
ethanol. The radioactivity was measured in a liquid
scintillation counter. SDS/PAGE and autoradiography
assessed the phosphorylated histone H1.
Immunocytochemistry
After HEK293 cells were cultured and transfected on
glass coverslips coated with poly
L
-lysine, cells were
washed twice in NaCl/P
i
and fixed for 30 min at room
temperature in 4% paraformaldehyde, NaCl/P
i
,and
10 m
M
EGTA washed and permeabilized (with 25 m
M
Tris, pH 7.4, 150 m
M
NaCI, and 0.2% Triton X-100) for
15 min. The coverslips were incubated overnight at 4 C
with primary antibodies: polyclonal anti-Cdk5 (C-8,
1 : 50), p35 (N-20 and C-19, 1 : 50), anti-cdc2 P34
(H-297 and 17, 1 : 50) antibodies; anti-tau, AT8
(1 : 500), anti-PS202 (1 : 250) and monoclonal TAU-5
mAb (1 : 100); monoclonal anti-T7.Tag antibody
(1 : 500) and Anti-Xpress–FITC (1 : 500). All antibodies
were diluted in NaCl/P
i
with 1% Triton X-100. After a
wash in NaCl/P
i
(3 ·15 min), the cells or coverslips were
incubated with 1 : 50 fluorescein isothiocyanate (FITC)-
conjugated goat anti-(mouse IgG) and rhodamine-labeled
goat anti-(rabbit IgG) secondary antibody for 1 h at
room temperature. This was followed by three washes
with NaCl/P
i
, and then the cells were embedded in
aqueous medium. Fluorescent images were observed with
a Zeiss LSM-410 laser-scanning confocal microscope.
Images were processed and merged by Adobe
PHOTOSHOP
software.
RESULTS
Identification of the Cdk5/p25 inhibitory peptide (CIP)
derived from p35
As discussed in the introduction, p35 residues 138–291 (p16)
are essential for effective Cdk5 activation and we found that
the peptide corresponding to p35 residues 154–279 (CIP)
(Fig. 1A,B) is a highly effective in vitro inhibitor of Cdk5
[20]. A dose–response relationship for CIP on Cdk5 activity
in vitro is shown in Fig. 1C. Cdk5 kinase activity was
determined by incubating various amounts of CIP with
Cdk5/p25 for 2 h at 30 C followed by incubation in the
kinase reaction mixture for an additional hour in the
presence of [c-
32
P]ATP and histone H1. Figure 1C shows
that the activity of Cdk5 is markedly inhibited in vitro by less
than 1 l
M
CIP.
CIP inhibits the activity of Cdk5 kinase
in transfected HEK293 cells
To explore the inhibitory effects of CIP on the Cdk5/p25
complex activity in vivo, we cotransfected HEK293 cells
with CIP and appropriate control expression constructs.
One set of transfections was carried out with the vector
alone (control), a second set was cotransfected with p25 and
Cdk5, and a third was cotransfected with p25, Cdk5 and
CIP. Cell lysates were subjected to Western blot analysis
using anti-Cdk5 (C-8) and anti-p35 (C-19) to detect the
expression of Cdk5 and p25 proteins, respectively. Anti-
Xpress–HRP that recognizes specifically constructed plas-
mids [24] was employed to detect the expression of CIP
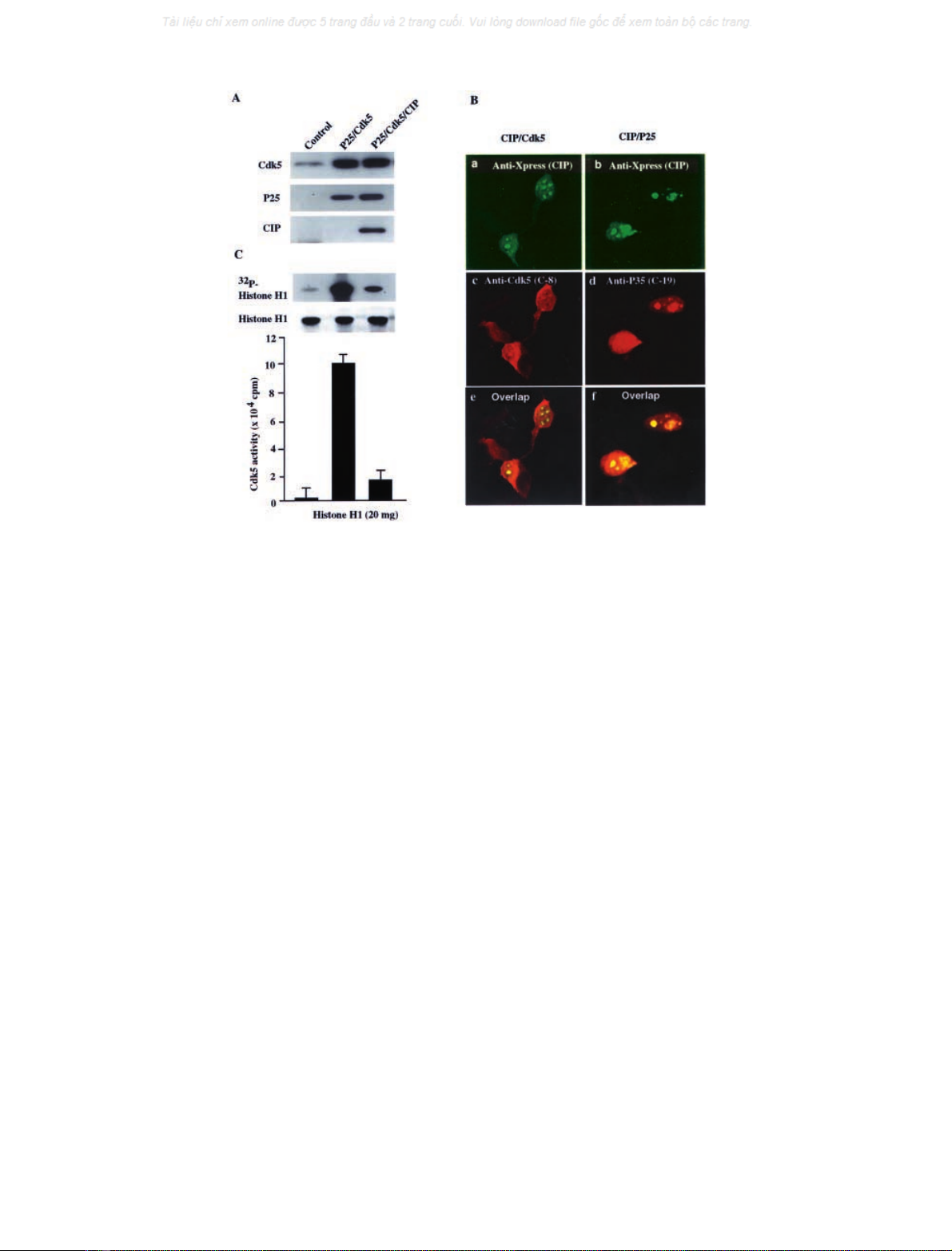
(Fig. 2A). We found that there is no endogenous p25 or
CIP, but endogenous Cdk5 is present in HEK293 cells
(Fig. 2A, left lane). There were clear bands of expression of
transfected Cdk5, p25 (Fig. 2A, middle and right lanes), and
CIP (Fig. 2A, right lane, bottom). These results were
confirmed by immunocytochemical analysis in transfected
cells (Fig. 2B). The antibodies used were anti-Xpress–FITC
(CIP), C-19-rhodamine (p35) and C-8-rhodamine (Cdk5).
There was clear expression of transfected CIP (Fig. 2B, a
and b), Cdk5 and p25 (Fig. 2B, c and d, respectively) in the
transfected cells.
To determine whether CIP inhibits the protein phospho-
rylation activity of Cdk5/p25 in transfected cells, kinase
activity assays were performed on the extracts. The activity
of Cdk5/p25 in cells transfected with the Cdk5/p25
constructs (Fig. 2C, middle lane) was markedly higher than
that in control cells. In comparison, the cells transfected
with Cdk5/p25 plus CIP was much lower (Fig. 2C, com-
pared middle and right lanes). Transfection of p25 alone
produced a threefold increase in Cdk5 activity compared to
control cells (no transfection), and was inhibited by
CIP transfection (data not shown). These results were
confirmed by kinase activity assays of anti-Xpress Ig
FEBS 2002 p35 peptide, CIP, inhibits tau phosphorylation (Eur. J. Biochem. 269) 4429
immunoprecipitates of the Cdk5/p25 and Cdk5/p25/CIP
complexes from the same cell lysates (data not shown).
These results demonstrate that CIP can substantially inhibit
Cdk5/p25 activity in transfected cells.
P25 phosphorylates tau more effectively than
p35 when Co-transfected with Cdk5
Cdk5 has been implicated, along with other kinases (e.g.
MAPK, GSK3, MARK), in the phosphorylation of tau
in transfected cells and mouse models of neurodegenera-
tive diseases [14,25–27]. If CIP inhibits the activity of
Cdk5/p25 as suggested by the above data, tau phospho-
rylation by Cdk5 might also be inhibited in CIP-trans-
fected cells. To compare CIP inhibitory effects on tau
phosphorylation, we first studied the effect of Cdk5/p25-
induced tau phosphorylation in cotransfected HEK293
cells (Fig. 3). We detected tau phosphorylation using
phospho-S202, a phospho-epitope-specific antibody [28].
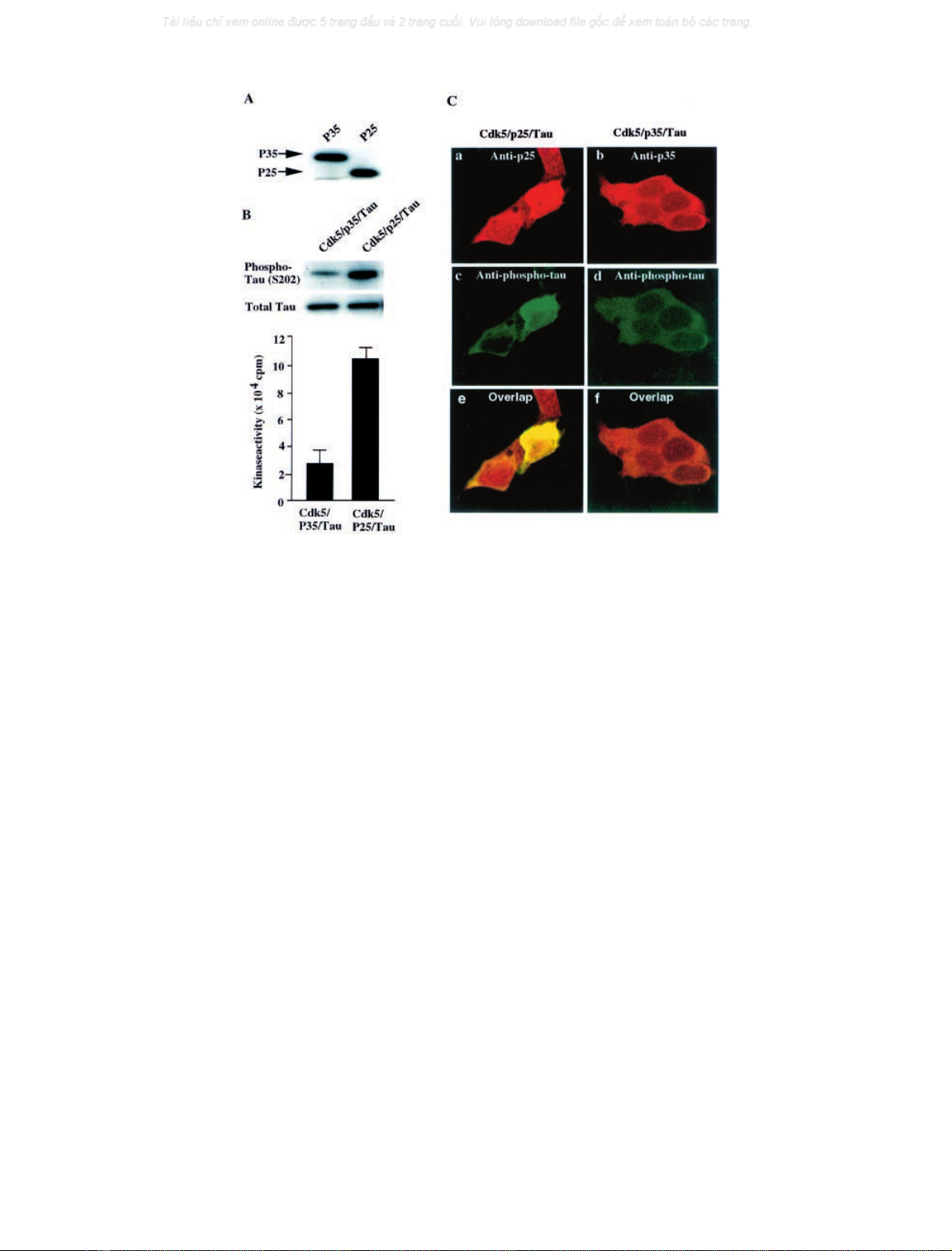
p35 and p25 were expressed at similar levels in the
transfected cells (Fig. 3A), but the tau phosphorylation in
the cells transfected with tau/p25/Cdk5 was markedly
higher than that in cells transfected with tau/p35/Cdk5
(Fig. 3B). The occurrence of more extensive phosphory-
lation of tau by Cdk5/p25 than by Cdk5/p35 is confirmed
by immunocytochemistry staining with the antitau
antibody, AT8. Again significantly increased tau
phosphorylation is shown to occur in cells transfected
with tau/p25/Cdk5 (Fig. 3C, c) compared with cells
transfected with tau/Cdk5/p35 (Fig. 3C, d). These results
agree with previous studies indicating that Cdk5/p25
causes tau hyperphosphorylation, whereas Cdk5/p35 does
not effectively phosphorylate tau in vivo [14,29].
CIP inhibits tau phosphorylation in cotransfected
HEK293 cells
Experiments by Patrick et al. showed by both Western blot
analysis and immunohistochemistry that p25 accumulates
in brains of patients with Alzheimer’s disease. They also
demonstrated that the Cdk5/p25 complex hyperphosphory-
lates tau in cultured neurons and is accompanied by
cytoskeletal disruption, morphological degeneration and
apoptosis [14].
Fig. 2. Analysis of Cdk5, p25, and CIP expression, and Cdk5 activity in transfected HEK293 cells. HEK293 cells were transiently transfected with the
following expression constructs: vector only; p25 with Cdk5; p25, Cdk5, CIP. (A) Western blot analysis of Cdk5, p25 and CIP expression. Forty-
eight hours after cotransfection of p25 and Cdk5 with or without CIP the cell lysates were prepared and subjected to Western blot analysis using
(from top to bottom) anti-Cdk5 (C-8), anti-p35 (C-19) (detecting p25) and anti-Xpress (detecting CIP) Ig. Equal amounts of protein were used in
each case. The left lane, control (vector only); the middle lane, Cdk5 and p25; and right lane, Cdk5, p25 and CIP transfected cells. (B)
Immunocytochemical analysis of CIP, Cdk5, and p25. Confocal micrographs illustrate the cells cotransfected with CIP (a and b), Cdk5 (c), and p25
(d). Cells were fixed and double-stained with anti-Xpress-FITC and polyclonal anti-Cdk5 (C-8) antibodies (a, c, and e) and with anti-Xpress-FITC
and polyclonal antip35 (C-19) antibodies (b, d, and f). Images were obtained using a Zeiss LSM 410 laser scanning confocal microscope. (C)
Analysis of Cdk5 kinase activity using in vitro kinase assays. After HEK293 cells were cotransfected with vector alone, p25 and Cdk5 with or
without CIP for 48 h, the cell lysates were immunoprecipitated with anti-Cdk5 (C-8) Ig and subjected to a kinase activity assay using histone H1 as a
substrate. The transfections of expression constructs were the same as shown in Fig. 2A. The left lane, control (vector only); the middle lane,
cotransfection of Cdk5/p25; the right lane,cotransfection of Cdk5/p25/CIP. Data represent mean ± SD of three experiments.
4430 Y.-l. Zheng et al. (Eur. J. Biochem. 269)FEBS 2002
To determine whether CIP can inhibit the hyperphos-
phorylation of tau, we cotransfected tau, p25, Cdk5, and
CIP constructs into HEK293 cells in the following four sets:
tau only, tau with Cdk5/p25, tau with p25/Cdk5/CIP, and
tauwithp25/Cdk5treatedwithroscovitine(10 lm), a Cdk5
inhibitor [30]. After 48 h transfection, the cells were lysed
and subjected to Western blot analysis. First, we used anti-
Cdk5 (C-8), anti-p35 (C-19), anti-Xpress-HRP, and anti-tau
(TAU-5) Ig to detect the levels of expression of transfected
Cdk5, p25, CIP, and tau, respectively. Transfected Cdk5
p25, CIP, and tau are clearly shown at 35, 25, 12.5, and
6.7 kDa., respectively (Fig. 4A). That Cdk5/p25 expression
significantly increases tau phosphorylation is evident by
both Western blot (Fig. 4B, lane 3) and immunofluores-
cence staining (Fig. 4C, b) with anti-(phospho-S202). To
observe whether tau phosphorylation decreases in trans-
fected cells in the presence of CIP, we detected tau
phosphorylation using tau phospho-S202 in Western blots
of these cell lysates. Cotransfection of CIP significantly
decreases tau phosphorylation in the cells (Fig. 4B, lane 2).
An inhibitor of Cdk5 (roscovitine) also decreases tau
phosphorylation (Fig. 4B, lane 4). These results are con-
firmed by immunocytochemical staining with anti-(phos-
pho-S202). The level of phosphorylated tau in the cells
cotransfected with CIP was significantly lower than that in
the cells cotransfected without CIP (Fig. 4C, compare a
with b). Thus, CIP can effectively decrease tau phosphory-
lation in Cdk5/p25 transfected cells.
CIP transfection does not inhibit Cdc2 activity
in HEK293 cells
Cdk5 is a member of cdc2-related kinase family [2] and cdc2
kinase is a nuclear protein that plays an essential function in
the normal cell cycle progression in eukaryotes. To assess
the specificity of CIP inhibition, we examined the effect of
CIP and roscovitine on cdc2 kinase activation in transfected
HEK293 cells (Fig. 5). The expressions of transfected CIP
and endogenous cdc2 protein in HEK293 cells are showed
by Western blots using anti-Xpress–HRP and anti-cdc2 p34
(17) Ig (Fig. 5A, upper and lower, respectively). cdc2 kinase
activity assays are performed using the same cell lysates. The
activities of cdc2 kinase were similar in nontransfected cells
(control, Fig. 5B, lane 1) and in cells transfected with CIP
(Fig. 5B, lane 3), but cells treated with roscovitine (10 l
M
)
had distinctly lower activity (Fig. 5B, lane 2, compared with
other lanes). These results indicate that CIP does not affect
cdc2 kinase activation, whereas roscovitine inhibits both
Cdk5 and cdc2 activity. To evaluate the specifity of anti-
cdc2 p34 (17) and anti-Cdk5 (C-8), we immunoprecipitated
the cell lysates of nontransfected (vector only) or cotrans-
fected with Cdk5/p25 using anti-Cdk5 or anti-cdc2 p34 (17)
Fig. 3. Phosphorylation of tau by Cdk5/p25 or Cdk5/p35 in transfected HEK293 cells. HEK293 cells were transiently cotransfected with rat tau
(181–242) and Cdk5 plus p25 or p35. (A) Analysis of p25 and p35 expression by Western blot using anti-p35 (C-19). Left lane, cotransfected with
Cdk5, p35 and tau; Right lane,cotransfected with Cdk5, p25 and tau. (B) Total tau and phospho-tau were analyzed by Western blot using anti-tau
(bottom) and phospho-tau S202 (top) Ig in aliquots of the same cell lysates. Left lane, cotransfected with Cdk5, p35 and tau; right lane,
cotransfected with Cdk5, p25 and tau. (C) Immunocytochemical analysis of p25, p35, and phospho-tau. After HEK293 cells were cotransfected
with Cdk5/p25/tau (left column) and Cdk5/p35/tau (right column) for 48 h, they were fixed and double-stained with polyclonal antip35 (C-19) and
anti-AT8 Ig (a, c, and e) and with polyclonal antip35 (N-20) and anti-AT8 Ig (b, d, and f). a and b expressed transfected p25 and p35, respectively;
c and d expresses phosphorylated tau. Images were obtained using a Zeiss (Thornwood, NY) LSM 410 laser scanning confocal microscope.
FEBS 2002 p35 peptide, CIP, inhibits tau phosphorylation (Eur. J. Biochem. 269) 4431



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





