
Role of CCP2 of the C4b-binding protein b-chain in protein S binding
evaluated by mutagenesis and monoclonal antibodies
Joanna H. Webb
1
, Bruno O. Villoutreix
2
, Bjo¨ rn Dahlba¨ck
1
and Anna M. Blom
1
1
Division of Clinical Chemistry, Department of Laboratory Medicine, Lund University, Sweden;
2
INSERM U428,
University of Paris V, France
Complement regulator C4b-binding protein (C4BP) and the
anticoagulant vitamin K-dependent protein S form a high
affinity complex in human plasma. C4BP is composed of
seven a-chains and a unique b-chain, each chain comprising
repeating complement control protein (CCP) modules. The
binding site for protein S mainly involves the first of the
three b-chain CCPs (CCP1). However, recently it has been
suggested that CCP2 of the b-chain also contributes to the
binding of protein S. To elucidate the structural background
for the involvement of CCP2 in the protein S binding,
several recombinant b-chain CCP1-2 variants having
mutations in CCP2 were expressed and tested for protein S
binding. Mutations were chosen based on analysis of a
homology model of the b-chain and included R60A/R101A,
D66A, L105A, F114A/I116A and H108A. All mutant pro-
teins bound equally well as recombinant wild type to pro-
tein S. Several monoclonal antibodies against the b-chain
CCP2 were raised and their influence on protein S binding
characterized. Taken together, the results suggest that the
role of CCP2 in protein S binding is to orient and stabilize
CCP1 rather than to be directly part of the binding site.
Keywords: binding site; C4BP; complement; protein S;
structure-function relationship.
C4b-binding protein (C4BP) is an important regulator of
the classical pathway of complement. C4BP also affects the
regulation of the coagulation system, as it binds protein S,
which serves as a cofactor to the anticoagulant activated
protein C [1]. C4BP and protein S form a high-affinity,
noncovalent 1 : 1 complex, the interaction being greatly
enhanced by calcium [2]. Only free protein S, which
accounts for approximately 30% of the total protein S in
plasma, can act as a cofactor to activated protein C [3],
whereas the functions of C4BP remain unperturbed when
C4BP is in complex with protein S [4]. We have recently
demonstrated that protein S can serve to localize C4BP to
the surface of apoptotic cells, C4BP retaining its ability to
bind complement protein C4b when attached to the
apoptotic cells surface [5]. C4BP has an octopus-like
structure being composed of seven elongated a-chains and
one shorter b-chain, the chains being held together by
hydrophobic forces that are stabilized by disulphide bridges
in the central core [6]. Each chain comprises several
complement control protein (CCP) domains, a CCP
domain being approximately 60 residues long containing
two disulphide bridges and a central antiparallel b-sheet [7].
It is the first of three CCPs (CCP1) of the unique b-chain
that contains the protein S binding site [8–11]. In this CCP,
a large hydrophobic patch is essential for binding of
protein S [12]. It has also been shown that CCP2 has a
moderate influence (approximately fivefold) on the interac-
tion between C4BP and protein S [13,14]. To investigate the
structural contribution of CCP2 to the C4BP–protein S
interaction, we have expressed several b-chain variants
carrying point mutations in CCP2 in a prokaryotic expres-
sion system and tested their ability to bind protein S. The
mutations introduced, R60A/R101A, D66A, L105A,
F114A/I116A and H108A, were chosen based on a
homology-based computer generated 3D-structure of the
C4BP b-chain [15]. In addition, we have raised and
characterized monoclonal antibodies against b-chain
CCP1-2 and tested their influence on the C4BP–protein S
interaction. None of the mutants affected the interaction
and taken together the results suggest that the role of CCP2
in the binding of protein S is to stabilize and orient CCP1
rather than to provide binding sites for protein S.
Materials and methods
Cloning procedure
Cloning of wild-type C4BP b-chain CCP1-2 has been
described previously [12]. This construct was then used as a
template and the mutations introduced using the Quik-
Change site-directed mutagenesis kit (Stratagene). Sense
primers used for mutagenesis were as follows (with template
used in parenthesis): R60A (wild-type b-chain CCP1-2) 5¢-
ACTGAGTGCGCCTTGGGCCACTGT-3¢, R60A/R101A
(R60A) 5¢-GGCAGCAATGCGAGCCAGTGTCTA-3¢,
D66A (wild-type b-chain CCP1-2) 5¢-CACTGTCCTGCTC
CTGTGCTG-3¢, L105A (wild-type b-chain CCP1-2) 5¢-AG
CCAGTGTGCAGAGGACCAC-3¢, F114A/I116A (wild type
b-chain CCP1-2) 5¢-GCACCTCCCGCTCCCGCCTGCA
Correspondence to B. Dahlba
¨ck, Division of Clinical Chemistry,
Department of Laboratory Medicine, Lund University,
University Hospital Malmo
¨, S-205 02 Sweden.
Fax: + 46 40 337044, Tel.: + 46 40 331501,
E-mail: Bjorn.Dahlback@klkemi.mas.lu.se
Abbreviations: C4BP, C4b-binding protein; CCP, complement
control protein; MoAb, monoclonal antibody; tPA, modified
plasminogen activator.
(Received 14 October 2002, accepted 14 November 2002)
Eur. J. Biochem. 270, 93–100 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03365.x

AAAGT-3¢, H108A (wild-type b-chain CCP1-2) 5¢-TGTCT
AGAGGACGCCACCTGGGCA-3¢. The various b–chain
constructs were then transformed into Escherichia coli DH5a
bacteria and mutations were confirmed using an automated
DNA sequencing (Perkin-Elmer).
Expression and purification of recombinant proteins
Recombinant proteins were expressed and purified essen-
tially as described before [12], with the exception of an
additional gel filtration purification step. Briefly, E. coli
strain BL21(DE3) transformed with the cDNA coding for
the recombinant proteins were induced with isopropyl thio-
b-
D
-galactoside to start expression of the proteins. Follow-
ing induction, the bacteria were sonicated and centrifuged
and the bacterial pellet was dissolved in a buffer containing
guanidine-HCl and reduced glutathione. The sample was
again sonicated and centrifuged, and the supernatant was
applied to a nickel-nitrilotriacetic acid Superflow column
(QIAGEN). Proteins were eluted from the column with a
buffer containing 100 m
M
EDTA. Fractions were chosen
and pooled based on measurement of absorbance at
280 nm. Dithiothreitol (100 m
M
) was added to the pooled
sample. Following reduction for 2 h at 4 C, the sample
was diluted in a buffer containing 3 m
M
cysteine and
0.3 m
M
cystine and refolding of the protein was accom-
plished by extensive dialysis against the same buffer. Free
cysteine residues were then blocked with iodoacetamide,
followed by dialysis against a buffer containing 10% (v/v)
glycerol. After dialysis the proteins were applied to a
MonoQ column (Amersham Pharmacia Biotech), fractions
were pooled after analysis of silver staining after SDS/
PAGE. Finally, all recombinant proteins were applied on a
gel filtration column (Superose 12 HR 10/30, Amersham
Pharmacia Biotech), previously equilibrated with 50 m
M
Tris/HCl, 150 m
M
NaCl (NaCl/Tris), pH 8.0. The flow rate
used was 0.5 mL per minute and 0.5 mL fractions were
collected. Fractions were chosen and pooled based on
analysis of silver staining after SDS/PAGE and stored at
)70 C until further use. Concentrations were determined
by measuring the absorbance at 280 nm, using an extinc-
tion coefficient (1%, 1 cm) of 10 for all recombinant
proteins.
Plasma purified proteins
C4BP and protein S were purified from human plasma, as
described before [16,17]. Protein concentration was deter-
mined by measuring absorbance at 280 nm. Extinction
coefficients (1%, 1 cm) used were 14.1 (C4BP) and 9.5
(protein S). Protein S was labelled with
125
Iusingthe
chloramine T method.
Monoclonal antibodies
Monoclonal antibodies (MoAb) 15 and 44 were raised using
a standard procedure, as described previously [18]. The
antigen used to immunize the mice was 20 lg per mouse per
injection of recombinant wild-type C4BP b-chain CCP1-2
[12]. Antibodies selected for subcloning were chosen by
ELISA. Twelve antibodies were purified using protein A
and protein G coupled columns (5 mL, Hi-trap, Amersham
Pharmacia Biotech). Antibody-containing cell medium was
applied to the columns, equilibrated with 20 m
M
NaPO
4
pH 7.0. The columns were then washed in the same buffer.
Bound antibodies were eluted with 0.1
M
glycine-HCl,
pH 2.7 with 1 mL fractions being collected in tubes
containing 50 lL Tris/HCl, pH 9.0. Fractions were pooled
after measurement of absorbance at 280 nm and dialyzed
against NaCl/Tris pH 8.0 (50 m
M
Tris/HCl, 150 m
M
NaCl). Following dialysis, the absorbance was again
measured and the concentration of antibody was calculated
using an extinction coefficient (1%, 1 cm) of 12.5. Anti-
bodies were stored at 4 C after addition of 0.02% NaN
3
.
Antibodies were tested using dot blot for their ability to bind
plasma purified C4BP. Two microlitres of serial dilutions of
C4BP were dotted on a nitrocellulose membrane. After
incubation with the purified antibodies (at 5 lgÆmL
)1
)
detection of binding was performed with a secondary rabbit
anti-mouse Ig (Dakopatt) conjugated with alkaline phos-
phatase and the membrane was developed. Two antibodies
showed clear recognition of plasma purified C4BP,
MoAb 15 and 44. We therefore decided to proceed with
more detailed analysis of these two antibodies. All mono-
clonal antibodies were tested for binding to three different
constructs. Two of the constructs were recombinant C4BP
a-chains where CCP1 or 2 had been replaced with their
b-chain counterpart (characterized in [9]). The third was the
recombinant molecule consisting of b-chain CCP1 and 2 (as
used for immunization of the mice). Each construct was
dotted on a membrane at equal concentration. After
incubation with the purified antibodies (at 5 lgÆmL
)1
)
detection of binding was performed with a secondary rabbit
anti-mouse serum (Dakopatts) conjugated with alkaline
phosphatase and the membrane was developed.
Electrophoretic and blotting techniques
Recombinant proteins. Recombinant proteins were separ-
ated on 15% SDS/PAGE, under reducing and nonreducing
conditions (approximately 1 lg) for the silver staining and
under nonreducing conditions for the radioligand blot
(approximately 1.5 lg). For the radioligand blot, the
proteins were transferred by electroblotting from the gel
to a poly(vinylidene difluoride)-membrane. The membrane
was then incubated for one hour at room temperature in a
quenching solution, composed of washing buffer [50 m
M
Tris/HCl pH 8.0, 150 m
M
NaCl, 0.5% (w/v) Tween 20]
supplemented with 3% fish gelatin. Then the buffer was
changed to washing buffer with 2 m
M
CaCl
2
and trace
amounts of
125
I-labeled protein S and the membrane
incubated for two hours at 4 C. The membrane was
further washed in washing buffer, dried and exposed in a
cassette. Finally the membrane was scanned using a
Phosphor-Imager (Molecular Dynamics).
Western blot. Western blot was used to test the binding of
MoAb 44 to recombinant wild-type b-chain and plasma
purified C4BP under both reducing and nonreducing
conditions. Varying amounts of protein were run on SDS/
PAGE (15% for recombinant wild-type b-chain, 5% for
unreduced plasma purified C4BP and 10% for plasma
purified reduced C4BP). The proteins were transferred from
the gel to a poly(vinylidene difluoride)-membrane. The
94 J. H. Webb et al.(Eur. J. Biochem. 270)FEBS 2003

membrane was then incubated for one hour at room
temperature in quenching solution. Following this, the
membrane was incubated with MoAb 44 or 15 (5 lgÆmL
)1
)
inthesamebuffer.Themembranewasthenwashedin
washing buffer before being incubated with the secondary
antibody (biotinylated goat anti-mouse, 2 lgÆmL
)1
)for
30 min. Following this, the membrane was washed again,
and incubated for 30 min with horseradish peroxidase
coupled Vectastain reagent (Vector Laboratories Inc., Ca,
USA) prepared according to the manufacturer’s recom-
mendations. Finally, the membrane was washed and
developed.
Protein S binding assays
Direct binding assay. Microtiter plates were coated with
50 lL of protein (wild-type or mutant recombinant b-chain)
at 2 lgÆmL
)1
in 75 m
M
Na-carbonate, pH 9.6, at 4 C
overnight. The plates were then washed three times in
washing buffer with 2 m
M
CaCl
2
and quenched for 1 h. The
plates were washed as above, and protein S added at
increasing concentrations (0–240 n
M
, final concentration),
plus trace amounts of
125
I-labeled protein S, at room
temperature for three hours. Finally, the plates were washed
five times in washing buffer and bound radioactivity
determined in a c-counter.
Competition assay. Microtiter plates were coated with
50 lL of plasma purified C4BP at 10 lgÆmL
)1
in 75 m
M
NaCO
3
,pH9.6,at4C overnight. The plates were then
washed three times in washing buffer and quenched in
washing buffer with 3% fish gelatin for 1 h, and washed as
before. Increasing amounts of plasma purified C4BP, or
recombinant b-chain (wild-type and mutants), plus trace
amounts of
125
I-labeled protein S were added overnight at
4C. The next day the plates were washed five times in the
same buffer as before and bound radioactivity was
measured in a c-counter.
Monoclonal antibody binding assays
Test of binding site for MoAbs. Microtiter plates were
coated overnight with 50 lL antigen (recombinant b-chain
CCP1-2 1 lgÆmL
)1
, plasma purified C4BP or C4BP–
protein S complex 10 lgÆmL
)1
)in75m
M
NaCO
3
,pH9.6,
at 4 C. Plates were washed three times in washing buffer
and blocked for 1 h at room temperature with NaCl/Tris
pH 7.5, 1% (w/v) BSA. Following blocking, serial dilutions
of MoAb 15 or 44 (0–20 lgÆmL
)1
) were added to the
immobilized C4BP for one hour at 37 C. Plates were again
washed three times, and incubated with secondary antibody
(goat anti-mouse serum coupled with horse radish peroxi-
dase) for 1 h at 37 C. Plates were then washed three times
before being developed and absorbance was read at 490 nm
in BioTek plate reader.
Test if protein S can bind recombinant C4BP b-chain in
the presence of MoAbs. Microtiter plates were coated
overnight with 50 lLofMoAb15or44at10lgÆmL
)1
in
75 m
M
NaCO
3
,pH9.6,at4C. Plates were washed and
blocked as above. Serial dilutions of wild-type recombinant
C4BP b-chain (0–500 n
M
) were added together with trace
amounts of
125
I-labeled protein S overnight at 4 C. The
next day, the plates were washed five times in the same
buffer as before and bound radioactivity was measured in a
c-counter.
Results
Selection of amino acids in CCP2 to be mutated
In this study, we were interested in investigating which
regions within CCP2 of the C4BP b-chain are involved in
protein S binding [13,14]. The mutagenesis strategy was
chosen based on the 3D-homology model of the C4BP
b-chain [15] (Fig. 1). A series of b-chain variants was created,
including R60A/R101A, D66A, L105A, F114A/I116A and
H108A. These changes were based on the theoretical analysis
and were expected to be structurally well tolerated. This was
also confirmed as recombinant mutant proteins were shown
to bind protein S with high affinity, which requires a b-chain
molecule with structural integrity. The purified recombinant
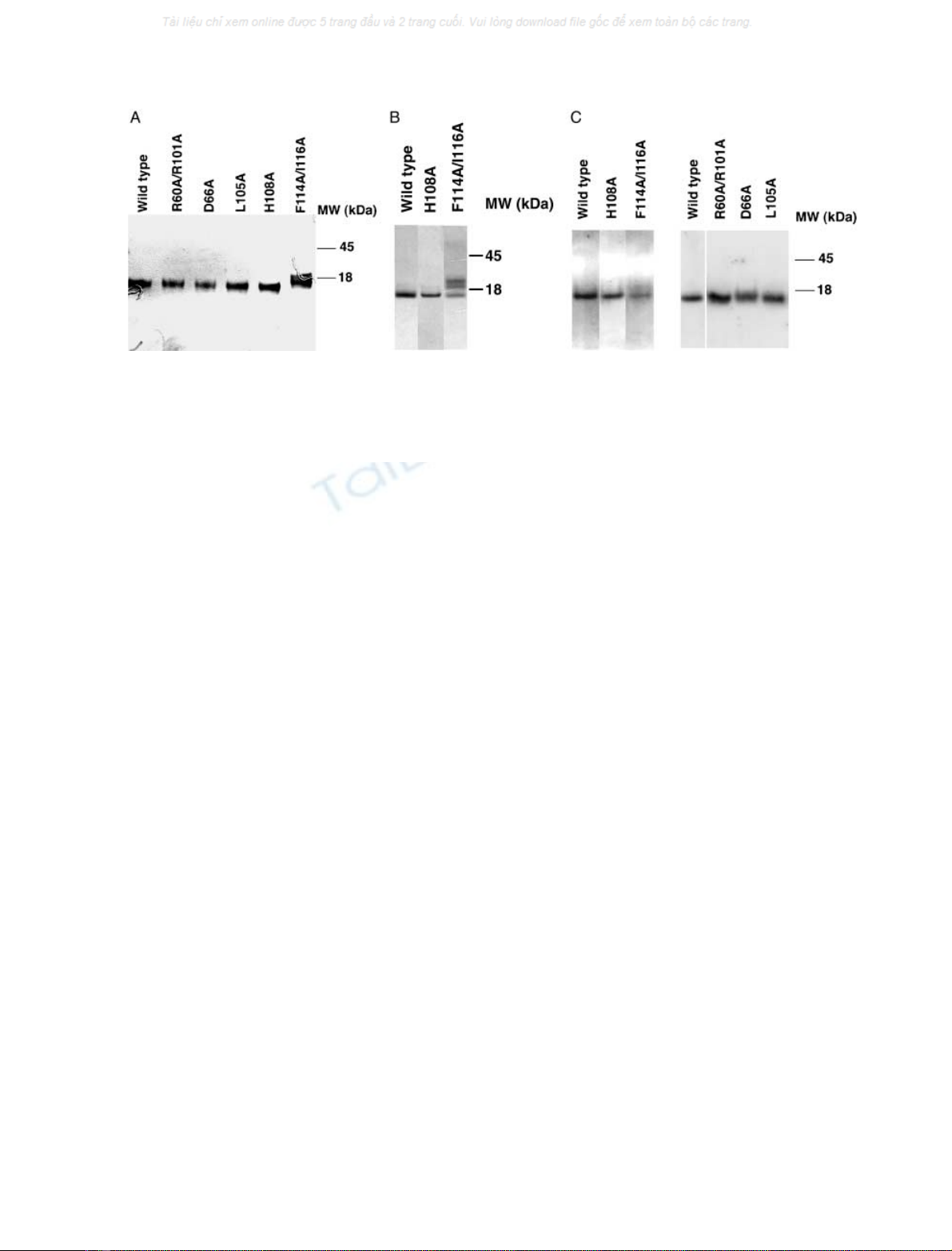
proteins were separated on SDS/PAGE under reducing
conditions (Fig. 2A). In addition, recombinant proteins with
the F114A/I116A and H108A mutations introduced were
separated on SDS/PAGE under nonreducing conditions
(Fig. 2B). All recombinant proteins looked very similar to
wild-type, except the variant carrying the F114A/I116A
mutation, which was present in more than one form.
Mutations in CCP2 had no effect on binding
of protein S
The b-chain constructs were tested in three different assays
for their ability to bind protein S. In the radioligand blotting
assay, proteins were separated by SDS/PAGE, blotted to a
poly(vinylidene difluoride)-membrane, and allowed to bind
125
I-labeled protein S in solution (Fig. 2C). All variants
yielded binding patterns similar to that of wild-type b-chain.
In the binding assay, the recombinant proteins were
immobilized in microtiter plates and tested for binding of
125
I-labeled protein S (Fig. 3A). The binding curves were
similar, with one exception. Mutant F114A/I116A showed
a slightly higher binding as compared to wild-type, with an
apparent twofold higher affinity at the most.
The b-chain variants were also tested for protein S
binding using a competition assay. Immobilized plasma
purified C4BP and fluid phase competitor (plasma purified
C4BP or recombinant wild-type or mutant b-chain) were
allowed to compete for binding of
125
I-labeled protein S
(Fig. 3B,C and Table 1). The apparent slightly lower
efficiency of the F114A/I116A variant (around threefold)
to compete for protein S binding was not statistically
significant. The results of the assay are dependent on the
concentration of correctly folded protein. On the silver
stained unreduced SDS/PAGE gel (Fig. 2B) it is apparent
that the F114A/I116A mutant is present in more than one
form. According to the radioligand blot (Fig. 2C), only one
of the F114A/I116A forms binds protein S. The results
suggest that the correctly folded F114A/I116A variant binds
protein S at least equally as well as the wild-type variant.
The apparent slightly decreased efficiency of the H108A
variant (around 1.5 times) in the competition assay was not
statistically significant.
FEBS 2003 The role of CCP2 of the C4BP b-chain in binding of protein S (Eur. J. Biochem. 270)95
Monoclonal antibodies
General. To further elucidate the influence of CCP2 on
protein S binding, we made the effort to raise monoclonal
antibodies against CCP1-2 with the aim to use them in
structure–function analysis. Two of 12 antibodies,
MoAb 15 and MoAb 44, recognized native C4BP purified
from plasma on a dot blot and were therefore selected for
detailed analysis. The remaining ten antibodies only
recognized the recombinant b-chain implying that access
to their epitopes was sterically hindered in the fully
assembled C4BP molecule. All antibodies were IgG,
MoAb 15 belonged to IgG1 and MoAb 44 belonged to
IgG2a class. The antibodies were tested on dot blot against
the previously characterized a–b-chain hybrids [9]. All the
antibodies reacted with constructs containing CCP2 and
MoAb 44 in addition gave a weak reaction against the
hybrids only containing CCP1 (results not shown). The
conclusion was that the epitopes of all antibodies were
mainly located in CCP2.
Western blot. MoAb 15 and MoAb 44 were tested for
their ability to recognize the recombinant wild-type b-chain
and plasma purified C4BP with Western blotting, under
reducing and nonreducing conditions. Both MoAbs recog-
nized the reduced b-chain of plasma purified C4BP and the
reduced recombinant CCP1-2. They also reacted with the
unreduced b-chain in purified C4BP from plasma and with
unreduced recombinant b-chain CCP1-2 (Fig. 4, only
MoAb 44 shown).
Binding assay. In an attempt to define the epitopes for the
MoAb 15 and MoAb 44, they were tested for binding of the
different recombinant b-chain CCP1-2 constructs. Two of
the b-chain variants, D66A and R60A/R101A, showed
weaker binding to both MoAbs (Fig. 5A,B). These two
variants bound protein S equally as well as the wild-type
b-chain, suggesting correct folding. It seems plausible to
conclude that the epitopes of both MoAb 15 and 44 involve
amino acids at mutated positions.
In binding to plasma purified C4BP that was immobilized
on microtiter plates, the two antibodies demonstrated a
difference because MoAb 15 bound whereas MoAb 44 did
not (Fig. 5C). However, for binding of MoAb 15 to occur,
the plates had to be coated with high concentrations of
C4BP (10 lgÆmL
)1
). When lower C4BP concentrations
were used, binding of MoAb 15 only reached approxi-
mately 10% (at highest concentration of MoAb 15) of the
binding seen to recombinant wild-type b-chain CCP1-2
(results not shown). This suggests that the multiple, long
a-chains to various extents sterically block the epitopes for
MoAb 15 (partial blockage) and MoAb 44 (complete
blockage) on the b-chain in immobilized C4BP.
Influence of protein S binding of monoclonal antibod-
ies. To investigate whether protein S and MoAb 15/
MoAb 44 had overlapping binding sites on the bchain,
the following four different binding assays using microtiter
plates were used. (a) First, the effect of increasing amounts
of fluid phase protein S on the binding of the MoAbs to
immobilized recombinant b-chain was tested. The binding
of both MoAb 15 and MoAb 44 to the b-chain was
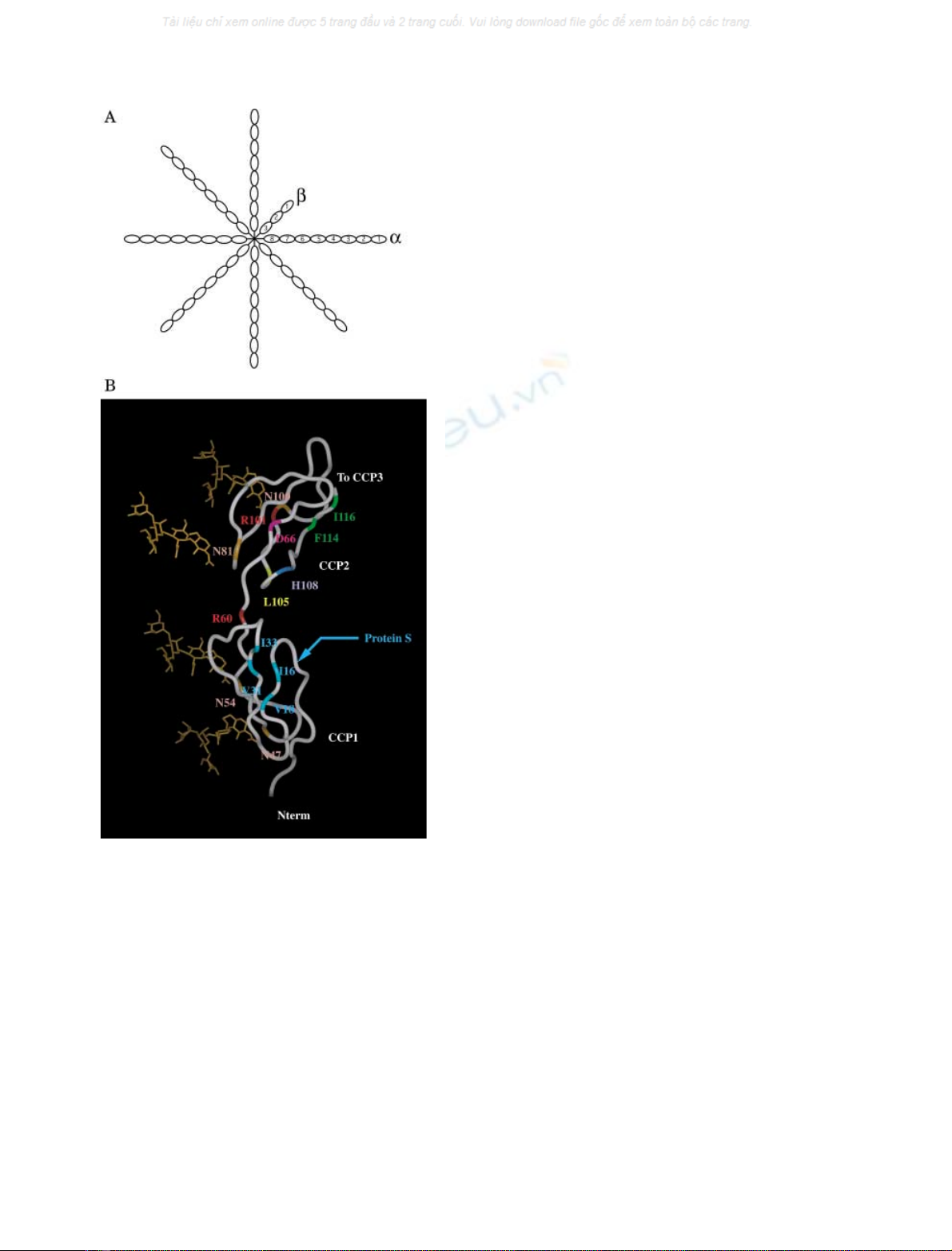
Fig. 1. Schematic outline of C4BP (A) and model of C4BP b-chain
CCP1-2 (B). (A) C4BP consists of seven identical a-chains and one
b-chain. Each chain is made up repeating complement control protein
(CCP) domains. The a-chains have eight such CCP domains, the
b-chain has three. All chains are held together by disulphide bridges
involving the nonrepeat carboxy-terminal regions. (B) A structural
model is shown as a ribbon diagram (adapted from [15]). Residues
expected to be glycosylated are shown in orange (N47, N54, N81 and
N100). The glycans are presented in orange but to illustrate the fact
that we neither know their exact size or orientation, they are not
attached to the protein. These glycans were positioned in a way con-
sistent with the covalent attachment to the molecule. Residues R60,
D66, R101, L105, H108, F114 and I116 were mutated in the present
study while key residues involved in protein S binding on CCP1 are
coloured blue (residues I33, V31, V18 and I16) [12].
96 J. H. Webb et al.(Eur. J. Biochem. 270)FEBS 2003
unaffected even by the highest concentration of protein S
used (240 n
M
, results not shown). (b) In the second
analysis, free C4BP and C4BP–protein S complexes were
immobilized and the binding of MoAb 15 tested (Moab 44
did not work in this assay system as described above).
MoAb 15 was found to bind equally well to both free and
complexed C4BP (Fig. 5D). (c) In the third assay, the
antibodies were immobilized and recombinant b-chain
added together with
125
I-labeled protein S. The
125
I-labeled
protein S–b-chain complex bound to MoAb 15 but not to
MoAb 44 (Fig. 6). In the MoAb 15 variant, the decreased
binding of
125
I-labeled protein S observed at higher
concentrations of b-chain suggested that free bchain
competes with the b-chain-protein S complex for binding
to the antibody (Fig. 6). (d) In the fourth assay, the effect of
MoAb 44 on the binding of protein S to immobilized
b-chain was investigated. In this assay protein S was found
to bind equally well in the absence and presence of
MoAb 44. Thus, from assays a and d it was concluded that
the immobilized b-chain was able to bind MoAb 44 and
protein S simultaneously. In contrast, immobilized
MoAb 44 could not bind the protein S–b-chain complex
(assay c). These results suggest that protein S and the two
MoAbs bind to different binding sites on the b-chain but
that under certain experimental conditions there was
sterical hindrance between protein S and MoAb 44.
Discussion
The binding site for protein S on C4BP is predominantly
contained in CCP1 of the b-chain [9]. A key binding surface
for protein S involves residues I16, V18, V31 and I33 on
CCP1 of the b-chain [12]. CCP2 has been shown to have a
small positive influence on the C4BP–protein S interaction
because a recombinant construct containing CCP1 but
lacking CCP2 had approximately five times lower affinity
for protein S than a construct containing both CCP1 and 2
[13]. In that study, chimeras between CCP domains from
the C4BP b-chain and the N-terminal of a modified
plasminogen activator (tPA) were used. The authors
concluded that the function of CCP2 was not simply to
serve as a spacer, which could be performed by any CCP
domain, but that CCP2 either induced a specific conform-
ational change in CCP1 or that it directly participated in the
binding of protein S. In an earlier study using recombinant
a–b-chain hybrids consisting of b-chainCCP1,1+2,and
1 + 2 + 3, we failed to observe this specific effect of CCP2
[9].
To address the discrepancy between the two studies, a
recombinant construct consisting of CCP1 from the C4BP
b-chain and CCP2 from the C4BP a-chain was fused to the
modified tPA [14]. The results of this exercise again
suggested b-chain CCP2 to have a specific positive influence.
To further evaluate the role of b-chain CCP2 in the
interaction with protein S we decided to introduce amino
acid changes into putative binding sites on b-chain CCP2.
The mutations were chosen based on a homology model of
the C4BP b-chain [15]. We have previously shown that a
solvent exposed hydrophobic patch on b-chain CCP1 was
crucial for binding protein S, and that electrostatic forces
played only a minor role [12,19]. The b-chain is heavily
glycosylated [20] but the glycans seem to have no role in
binding of protein S because prokaryotic recombinant
b-chain constructs bind equally well to protein S as plasma
purified C4BP. Thus, with the structural information gained
from our model and experimental data, and general
knowledge of protein–protein interactions, we could select
areas potentially involved in the binding of protein S. We
were interested in mutating residues close to the CCP1–
CCP2 interface and amino acids of hydrophobic nature.
Yet, it was also important to assess the role of charged or
polar residues, and of amino acids located away from the
intermodule region. In the present situation, three param-
eters could not be well defined; the exact angle between the
CCP modules (assuming there is such an angle between
CCP modules), which Asn residue is glycosylated and the
exact orientation of the glycans relative to the protein core
(suchachaincouldbeflexible).
Fig. 2. SDS/PAGE analysis of recombinant C4BP b-chain CCP1-2. (A) Indicated recombinant proteins (wild-type and mutants), approximately
0.5 lg per well, were separated on SDS/PAGE under reducing conditions. Proteins were visualized using silver staining. (B) Selected recombinant
proteins (wild-type and mutants), approximately 1 lg per well, were separated on SDS/PAGE under nonreducing conditions. Proteins were
visualized using silver staining. (C) Recombinant proteins (wild-type and mutants), approximately 1.5 lg per well, were separated on SDS/PAGE
under nonreducing conditions. The proteins were then transferred to a poly(vinylidene difluoride)-membrane and incubated with
125
I-labeled
protein S. Bound protein S was detected using a PhosphorImager.
FEBS 2003 The role of CCP2 of the C4BP b-chain in binding of protein S (Eur. J. Biochem. 270)97


























