
A novel retinol-binding protein in the retina of the swallowtail
butterfly,
Papilio xuthus
Motohiro Wakakuwa
1
, Kentaro Arikawa
1
and Koichi Ozaki
2
1
Graduate School of Integrated Science, Yokohama City University, Yokohama, Kanagawa;
2
Graduate School of Frontier Biosciences,
Osaka University, Toyonaka, Osaka, Japan
Retinoid-binding proteins are indispensable for visual cycles
in both vertebrate and invertebrate retinas. These proteins
stabilize and transport hydrophobic retinoids in the hydro-
philic environment of plasma and cytoplasm, and allow
regeneration of visual pigments. Here, we identified a novel
retinol-binding protein in the eye of a butterfly, Papilio
xuthus. The protein that we term Papilio retinol-binding
protein (Papilio RBP) is a major component of retinal
soluble proteins and exclusively binds 3-hydroxyretinol, and
emits fluorescence peaking at 480 nm under ultraviolet (UV)
illumination. The primary structure, deduced from the
nucleotide sequence of the cDNA, shows no similarity to any
other lipophilic ligand-binding proteins. The molecular mass
and isoelectric point of the protein estimated from the
amino-acid sequence are 26.4 kDa and 4.92, respectively.
The absence of any signal sequence for secretion in the
N-terminus suggests that the protein exists in the cytoplas-
mic matrix. All-trans 3-hydroxyretinol is the major ligand of
the Papilio RBP in dark-adapted eyes. Light illumination of
the eyes increases the 11-cis isomer of the ligand and induces
redistribution of the Papilio RBP from the proximal to the
distal part of the photoreceptor layer. These results suggest
that the Papilio RBP is involved in visual pigment turnover.
Keywords: retinol-binding protein; rhodopsin; visual pig-
ment; visual cycle.
Retinalaldehyde (retinal) plays an essential role in animal
vision as the chromophore of visual pigments that are
generically called rhodopsins. In the rhodopsin molecule,
retinal is bound to the protein, opsin, in the 11-cis
configuration. Light energy first isomerizes the chromo-
phore into its all-trans form that subsequently causes a
conformational change of the opsin into an active form. The
activated rhodopsin, usually called metarhodopsin, triggers
the phototransduction cascade, that eventually controls the
flow of ion currents through cation channels in the plasma
membrane of the photoreceptor cell. Prolonged illumination
will cause depletion of rhodopsin unless its chromophore is
replenished. An important pathway for rhodopsin replen-
ishment in all known photoreceptor cells is the recovery of
all-trans retinal from opsin, its reverse isomerization to the
11-cis form, and subsequent recombination with opsin.
Some processes in the pathway do not occur in the
photoreceptive membrane, where rhodopsin molecules
are embedded and function. Thus, the retinal has to
be transported, when necessary, in hydrophilic matrices.
As retinoids are highly hydrophobic and hardly soluble in
water, hydrophilic retinoid-binding proteins are therefore
required for stabilizing retinoids in the watery plasma as
well as in the cytoplasm, and for transporting retinoids
within and/or between cells [1]. In addition, recent studies
have demonstrated that such protein is not simply a carrier
of retinoid. Regulation of retinoid concentration and its
delivery to various cells, protection of retinoid from
degradation and protection of cells from the potentially
toxic properties of free retinoid may also be biologically
important functions of retinoid-binding proteins (reviewed
in [2]).
The rhodopsin recycling system, the visual cycle, is well
characterized in vertebrates (reviewed in [3–5]). Briefly, all-
trans retinol bound to serum retinol-binding protein (RBP)
circulates in the blood and is targeted to the retinal pigment
epithelial (RPE) cells. There it is possibly transferred to
cellular retinol-binding protein (CRBP) and esterified to all-
trans-retinyl ester. After hydrolysis and isomerization to
the 11-cis form, it is transferred to cellular retinal-binding
protein (CRALBP) and oxidized to 11-cis retinal. Several
mechanisms for the isomerization from all-trans to 11-cis
isomer have been proposed. These include coupling of the
hydrolysis of all-trans-retinyl esters to isomerization gener-
ating 11-cis-retinol [6], or the presence of an enzyme
catalyzing the direct isomerization of all-trans-to11-cis-
retinol through a carbocation intermediate [7]. In both
cases, the isomerization requires the presence of CRALBP
[6,7]. Another pathway for isomerization is mediated by
RPE retinal G-protein-coupled receptor (RGR). RGR is a
vertebrate homolog of squid retinochrome (see below), and
catalyzes light-dependent isomerization of all-trans-to
11-cis-retinal [5,8]. The 11-cis-retinal formed in the RPE
cells is then transported across the interphotoreceptor
Correspondence to K. Ozaki, Graduate School of Frontier
Biosciences, Osaka University, 1-1 Machikaneyama, Toyonaka,
Osaka 560-0043, Japan. Fax/Tel.: + 81 6 6850 5439,
E-mail: ozaki@bio.sci.osaka-u.ac.jp
Abbreviations: CRALBP, cellular retinal-binding protein; CRBP,
cellular retinol-binding protein; IRBP, interphotoreceptor
retinoid-binding protein; RBP, retinol-binding protein.
Note: The nucleotide sequence reported in this paper has been
deposited in the DDBJ/EMBL/GenBank under the accession number
AB070628.
(Received 12 February 2003, revised 4 April 2003,
accepted 9 April 2003)
Eur. J. Biochem. 270, 2436–2445 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03614.x

matrix to the photoreceptor cells. Involvement of inter-
photoreceptor retinoid-binding protein (IRBP) in this
step has been advocated, but is, however, still in dispute
(reviewed in [2]). In photoreceptor cells, retinal binds to
opsin to form rhodopsin. All-trans-retinal, liberated from
opsin after light absorption, is reduced into all-trans-retinol
in the photoreceptor cells and then moved back to retinal
pigment epithelial cells.
Regeneration of rhodopsin in invertebrates is somewhat
different from that of invertebrates, as studied intensively in
cephalopods and insects. Metarhodopsins of these animals
are usually thermostable, i.e. the opsin and the chromo-
phore do not immediately separate as they do in vertebrates.
Therefore, metarhodopsins can absorb light whose wave-
length is different from the wavelength absorbed by
rhodopsins. Upon light absorption by metarhodopsin, all-
trans-retinal is reconverted to 11-cis form, and thus,
rhodopsin is regenerated. This pathway is called photo-
reconversion or photoregeneration. In addition to this
photochemical reaction, there exists another pathway
through which rhodopsin is metabolically regenerated
(visual cycle). In squid, Todarodes pacificus, metarhodopsin,
resulting from photoconversion of rhodopsin, transfers
its all-trans-retinal to squid retinal-binding protein (squid
RALBP) [9]. The protein transports the all-trans-retinal
from the outer segment to the inner segment of the
photoreceptor cell [10,11]. In the inner segment, all-trans
retinal is transferred to retinochrome. Light absorption by
the retinochrome-all-trans-retinal complex causes photo-
isomerization of the all-trans-retinal to the 11-cis form,
which is then transferred to the squid RALBP and sub-
sequently transported back to the outer segment. The squid
RALBP provides the attached 11-cis-retinal to metarho-
dopsin and, in return, receives all-trans-retinal: the rhodop-
sin is thus regenerated. In this system, squid RALBP
functions as a shuttle carrying 11-cis- and all-trans-retinal
back and forth between the inner and the outer segments
[10,12]. A similar recycling system using retinochrome and
RALBP is also found in gastropods [13,14]. Recently,
Robles et al. suggested the direct interaction of rhodopsin
with retinochrome, based on immunocytochemical obser-
vations [15]. However, this finding does not completely rule
out the involvement of RALBP in chromophore transport
in the cephalopod visual cycle.
The visual cycle in insect retina has been studied in several
species. In the blowfly retina, metarhodopsin is degraded
slowly into opsin and all-trans-3-hydroxyretinal [16]. HPLC
analysis of retinoids suggested that the liberated all-trans-3-
hydroxyretinal might be bound to a protein that mediates
photoisomerization of the all-trans-3-hydroxyretinal to the
11-cis form [17,18]. A protein having required properties has
been isolated from the honeybee retina [19,20], but not yet
from fly. The 11-cis-3-hydroxyretinal is then reduced to
alcohol (11-cis-3-hydroxyretinol) followed by slow re-oxi-
dation to aldehyde (11-cis-3-hydroxyretinal). The aldehyde
would be used as a chromophore to regenerate rhodopsin.
Involvement of 11-cis-3-hydroxyretinol in this pathway was
proposed based on the observation that the amount of
11-cis-3-hydroxyretinol was increased considerably by light-
adaptation [17]. Also in the butterfly retina, it has been
demonstrated that metarhodopsin is degraded rapidly [21],
and abundant 3-hydroxyretinol is contained in the soluble
fraction [22,23]. These findings suggest that a visual cycle
similartothatintheflyalsoexistsinthebutterflyretina.
In addition, it was demonstrated in the Japanese yellow
swallowtail butterfly, Papilio xuthus, that the isomer com-
position of the 3-hydroxyretinol changes between the light-
and dark-adaptation, suggesting that the 3-hydroxyretinol is
possibly involved in the visual cycle. Although these studies
strongly suggest that some retinol-binding protein may be
involved in the insect visual cycle, no such a protein has been
identified.
In addition to the above biochemical studies, we recently
found that the Papilio compound eye consists of three
distinct types of ommatidia, one of which emits strong
fluorescence under ultraviolet light [24]. The microspectro-
fluorometric study suggested that the fluorescence is due to
3-hydroxyretinol that can act as a UV absorbing spectral
filter. These previous observations suggested strongly that
some kind of retinol-binding protein possibly localized in
the Papilio retina, and functions in the visual cycle and/or
color vision.
In this study, we therefore isolated a soluble retinol-
binding protein from the Papilio retina, and performed
molecular biological and biochemical analyses of the pro-
tein. As the protein is a novel species of the hydrophobic-
ligand-binding protein and solely binds 3-hydroxyretinol
as an intrinsic ligand, we termed this protein the Papilio
retinol-binding protein (Papilio RBP). Further analysis
suggested that Papilio RBP is involved in the visual cycle
rather than the ommatidial fluorescence.
Materials and methods
Animals
We used both sexes of the Japanese yellow swallowtail
butterfly, Papilio xuthus Linnaeus. The butterflies were
reared on fresh citrus leaves at 25 C under a light regime of
8-h light : 16-h dark. The pupae were stored at 4 Cfor
atleast3monthsandthenallowedtoemergeat25C.
When necessary, the butterflies were dark-adapted for 48 h
in complete darkness, or light-adapted for 12 h by posi-
tioning the animals 5-cm from a 15 W white fluorescent
lamp. For light-adaptation, butterflies were immobilized by
clipping their wings and fixed in appropriate positions.
Column chromatography
Papilio RBP was purified from a water-soluble fraction of
the retina by two-step column chromatography. All of the
following procedures were conducted under dim red light.
Retinas of the dark-adapted butterflies were detached from
the corneal cuticle of the compound eyes and homogenized
in 63 m
M
Tris/HCl buffer (pH 6.8). The homogenate was
centrifuged at 15 000 gfor 15 min at 4 C yielding a clear
supernatant containing only soluble proteins. The proteins
in the extract were first separated by anion-exchange
chromatography using the SMART System (Amersham
Pharmacia Biotech) equipped with a Mono Q column that
was equilibrated with 20 m
M
bis/Tris/HCl buffer (pH 6.5)
at room temperature. The proteins were eluted with a linear
gradient from 0–0.4
M
NaClinthesamebuffer.The
fractions that emit bluish fluorescence under UV-irradiation
FEBS 2003 Retinol-binding protein in butterfly eye (Eur. J. Biochem. 270) 2437

were collected, concentrated by ultrafiltration, and subjected
to further purification using size-exclusion chromatography.
The chromatography was performed using the SMART
System equipped with a Superdex 75 column. The column
was equilibrated with 150 m
M
bis/Tris/HCl (pH 6.5) con-
taining 0.15
M
NaCl, and proteins were eluted with the same
buffer at room temperature. The absorbance of the eluent
was monitored at 280 nm and 330 nm.
Gel electrophoresis
Besides the column chromatography, native PAGE was
also used for purification of Papilio RBP as follows. The
compound eyes were homogenized in 63 m
M
Tris/HCl
buffer (pH 6.8), and the homogenate was centrifuged at
15 000 gfor 30 min at 4 C. The supernatant was put on a
5% polyacrylamide concentrating gel (125 m
M
Tris/HCl,
pH 6.8), and proteins in the supernatant were separated in
a 10% polyacrylamide gel (375 m
M
Tris/HCl, pH 8.8)
under electrophoresis using Tris/glycine (25/192 m
M
) run-
ning buffer. After electrophoresis, the gel was illuminated
with UV light that visualizes a single band of Papilio RBP
by strong whitish fluorescence. A piece of gel containing
the fluorescing band was then cut out, and Papilio RBP
was eluted electrophoretically out of the gel. Alternatively,
the gel was placed in a whole gel elutor (Bio-Rad)
immediately after electrophoresis, and fluorescing fractions
were retrieved electrophoretically. Regular SDS/PAGE
was also performed according to Laemmli (1970) by the
use of 12% polyacrylamide gel [25]. The gel was then
stained with Coomassie Brilliant Blue to visualize the
proteins.
Protein digestion and sequencing
Papilio RBP was purified from 100 compound eyes as
described above. The purified protein was digested with
10 pmol of lysyl-endopeptidase in 83 m
M
Tris/HCl buffer
(pH 9.2) for 5 h at 37 C. The reaction was stopped by
adding trifluoroacetic acid to the reaction mixture at a final
concentration of 0.04%. Peptides were separated and
isolated by reverse-phase HPLC (SMART System) using
alRPC C
2
/C
18
column equilibrated with 0.1% trifluoro-
acetic acid. Peptides were eluted with a 0–80% linear
gradient of acetonitrile containing 0.1% trifluoroacetic acid.
Elution was monitored at 215 nm and peaks were collected
separately. Amino acid sequences of isolated peptides were
determined using a protein sequencer (Model G1005A,
Hewlett Packard). For nucleotide sequencing of Papi-
lio RBP cDNA, Poly(A)
+
RNA was prepared from 40
compound eyes using a QuickPrep mRNA Purification Kit
(Amersham Pharmacia Biotech), and used for synthesis of
cDNA with oligo(dT) primer. We prepared three pairs of
oligo nucleotide primers (ROLBP1-forward, 5¢-AARGAR
GAYGTNTGG-3¢; ROLBP1-reverse, 5¢-CCANACRTC
YTCYTT-3¢; ROLBP2-forward, 5¢-AARGCNGGNAT
HYT-3¢; ROLBP2-reverse, 5¢-ARDATNCCNGCYTT-3¢;
ROLBP3-forward, 5¢-AARGTNTGGWSNGA-3¢;ROLB
P3-reverse, 5¢-TCNSWCCANACYTT-3¢)basedonthe
amino acid sequences determined above (KEDVW, KAG
IL, KVWSE). Using these primers, we amplified the Papilio
retinal cDNA by PCR, and determined the nucleotide
sequences of amplified cDNA products. The 3¢-and
5¢-RACE were employed to complete sequencing of the
entire coding region of the Papilio RBP cDNA. For 3¢-
RACE, the primer containing EcoRI–SacI–KpnIsites
and poly(T) sequences (ROLBP-RT1, 5¢-GCCGAATT
CGAGCTCGGTACCTTTTTTTTTTTTTTTTT-3¢)was
prepared to synthesize the first strand cDNA from the
Papilio retinal mRNA. Based on the nucleotide sequence of
the above PCR products, specific forward primers (ROL-
BP4-F, 5¢-TTGCTTCCTCACGGCACCAG-3¢; ROLBP5-
F, 5¢-GACTAGTGGTGAACATGTGTATGCCGCAG-
3¢) were synthesized and used for PCR with the first strand
cDNA (template) and the partial sequence of ROLBP-RT1
(T-RAP, 5¢-GCCGAATTCGAGCTCGGTACC) as a
reverse primer. To synthesize the first strand cDNA for
5¢-RACE, a specific reverse primer (ROLBP-RT2,
5¢-TCTGCTCAATGATTGATGTC-3¢) was prepared.
The poly(A) sequence was attached to the 5¢-end of the
cDNA, which was then amplified by PCR, using a set of
primers, ROLBP-RT1 and ROLBP7-R (5¢-GACTAG
TATCGCTTCAGGGTCCTCCGCTG-3¢). The product
was again amplified with the second set of primers,
T-RAP and ROLBP7-R.
Ligand analysis
The ligand analysis experiments were carried out under dim
red light. Ten compound eyes were used for each experi-
ment. In order to analyze the geometric isomers of retinoids
using HPLC, Papilio RBP was isolated by native PAGE
from the crude extract of the light-adapted or dark-adapted
retinas, and finally dissolved in 200 lL PAGE running
buffer. Each sample (200 lL) was mixed with 60 lLof2
M
hydroxylamine (NH
2
OH) and 400 lLofcold90%meth-
anol to convert 3-hydroxyretinals, if any, to retinaloximes.
Retinoids and retinaloximes were extracted with 500 lL
dichloromethane and 6 mL n-hexane. The extract was then
concentrated and separated with a Hitachi model 635
HPLC system equipped with a YMC A-012 column (5-lm
silica gel, 6 ·150 mm, Yamamura Chemical Laboratory).
Elution was carried out with n-hexane containing 25% ethyl
acetate and 2% ethanol at a flow rate of 1.2 mLÆmin
)1
,and
the eluent was monitored for absorbance at 340 nm. In this
elution condition, isomers of 3-hydroxyretinaloximes and
3-hydroxyretinol were eluted between 10 and 35 min, while
isomers of retinaloximes and retinol were eluted just after
the solvent front without enough resolution. In the present
study, we did not carry out further analysis of retinaloxims
and retinol, as neither retinal nor retinol are contained in the
Papilio retina [22]. Standard isomers of 3-hydroxyretinal
were synthesized by M. Ito (Kobe College of Pharmacy,
Japan) [22]. Isomers of 3-hydroxyretinol were prepared
by reducing the corresponding isomers of 3-hydroxyretinal
in ethanol with a trace amount of sodium borohydride.
For routine analyses, isomers of 3-hydroxyretinal and
3-hydroxyretinol extracted from Drosophila heads were also
used as a standard mixture. The molar ratio of retinol
isomers was calculated by using their extinction coefficients
at 340 nm in the eluent (all-trans, 39 100; 11-cis, 22 700;
13-cis, 42 500). In order to measure absorption and
fluorescence spectra of Papilio RBP, the fluorescing protein
was collected from dark-adapted compound eyes using a
2438 M. Wakakuwa et al. (Eur. J. Biochem. 270)FEBS 2003
whole gel elutor as described above, dialyzed to remove
acrylamide contamination, concentrated with a Centricon
YM-10 (Millipore), and re-dissolved in 10 m
M
Tris/HCl
(pH 8.0) buffer. Absorption and fluorescence spectra were
measured with a Hitachi model U-3300 spectropho-
tometer and a Hitachi model F-4500 spectrofluorometer,
respectively.
Localization of
Papilio
RBP in light- and dark-adapted
eyes
Light- or dark-adapted Papilio retina was divided into distal
and proximal portions by pulling out the retina from the
corneal cuticle. This manipulation allows the eyes to be
separated into the distal portion, which contains distal one-
third of the photoreceptor layer in addition to the cornea
and the crystalline cone, from the rest that we call the
proximal portion. After performing native PAGE (see
above) in the distal and proximal portions separately, we
compared the fluorescence intensity between the portions on
the gel. The fluorescence intensity was measured directly
with a CCD camera, stored using an ATTO AE6905C
Image Saver, and quantified with
NIH IMAGE
program. The
gel was then stained with Coomassie Brilliant Blue, and
the protein content was measured via the absorbance of the
stained bands using a Sharp JX-350 image scanner. We
further analyzed the isomer composition of the intrinsic
ligands of Papilio RBP extracted separately from the distal
and proximal portions of the retina. Papilio RBP was
extracted from each portion of the dark-adapted or light-
adapted retina, and purified by native PAGE. The ligand
was then analyzed by HPLC as described above.
Results
Purification of
Papilio
RBP
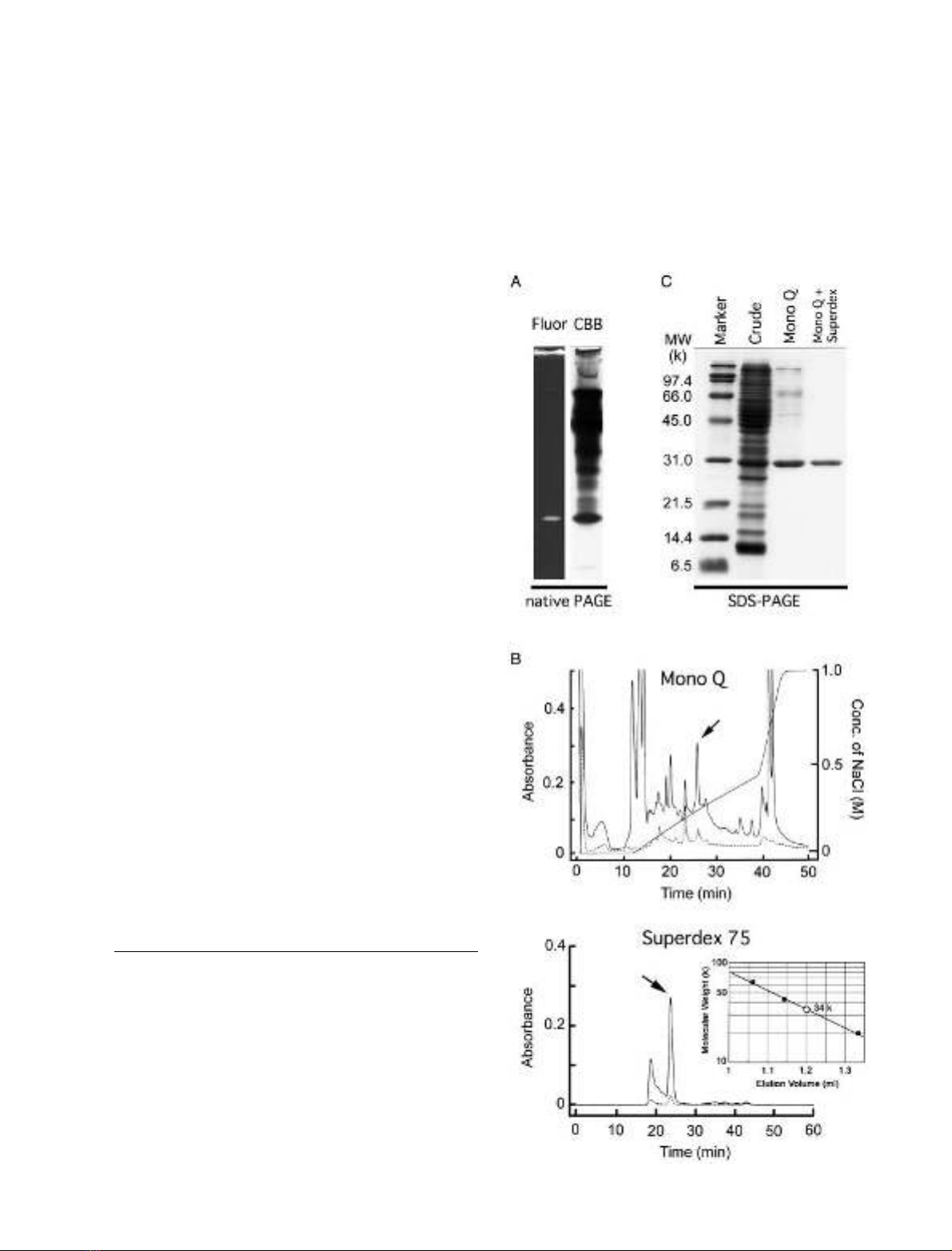
Figure 1A shows the results of native PAGE of crude
extract from Papilio compound eyes. We identified a single
band emitting whitish fluorescence under UV illumination.
Coomassie Brilliant Blue staining of the gel indicates that
the fluorescing protein is one of the major components of
soluble proteins in the crude extract. The surface of the
fluorescing protein carries negative charge in total, because
the protein expresses high mobility in the native gel.
We purified the fluorescing protein from the gel by
two-step column chromatography. We first separated the
crude extract with an anion-exchange (Mono Q) column
and then with a size-exclusion (Superdex 75) column
(Fig. 1B). With this purification procedure, we isolated the
protein from other soluble proteins, shown as a single band
in a SDS/PAGE gel (Fig. 1C). The apparent molecular
mass of this protein was 31 kDa on the SDS/PAGE gel,
which was close to 34 kDa estimated from the size-exclusion
chromatography in the native state (Fig. 1B, inset).
Fig. 1. Purification of Papilio RBP. (A)NativePAGEofthecrude
extractofthePapilio retina. Fluorescence under UV (left) and Coo-
massie Brilliant Blue (CBB) staining (right). (B) Anion exchange
(Mono Q, top) and size-exclusion (Superdex 75, bottom) chromato-
graphs of Papilio RBP. The fluorescent fraction (arrow) in the anion
exchange chromatography was collected, and re-chromatographed
with Superdex 75 column. A well-separated fluorescent peak of Papi-
lio RBP (arrow), whose molecular mass is estimated to be approxi-
mately 34 kDa (open circle in inset) was isolated. During
chromatography, eluents were continuously monitored via light
absorption at 280 nm (solid lines) and 330 nm (dotted lines). (C) SDS/
PAGE analysis of the crude extract and the purified Papilio RBP.
FEBS 2003 Retinol-binding protein in butterfly eye (Eur. J. Biochem. 270) 2439
This suggests that the protein exists in a monomeric state
in vivo.
Biochemistry of
Papilio
RBP
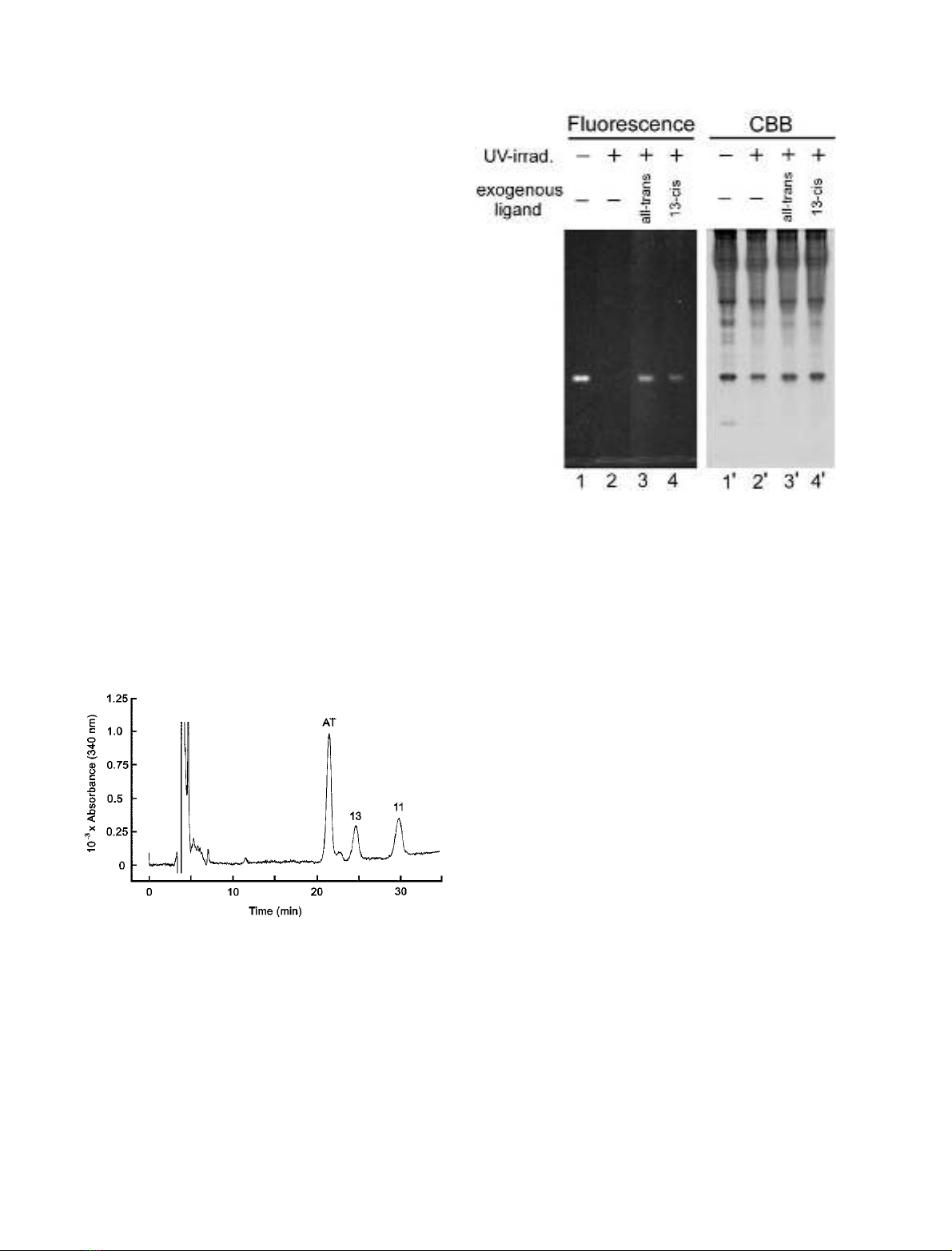
To determine the native ligand of the fluorescing protein, we
extracted retinoids from the purified fluorescing protein
collected from dark-adapted Papilio eyes, and analyzed the
composition of retinoids with HPLC (Fig. 2). It appeared
that the protein exclusively binds 3-hydroxyretinol. We
therefore call the protein Papilio retinol-binding protein, or
Papilio RBP. HPLC analysis demonstrated that protein
prepared from dark-adapted animals contained the all-trans
isomer as the major ligand, but significant amounts of 11-cis
and 13-cis isomers were also detected.
The UV-induced fluorescence of the Papilio RBP disap-
peared after the intense UV-irradiation, probably because
the ligand was degraded. To investigate whether Papi-
lio RBP has the ability to bind exogenous retinol, we
supplied, after irradiating the soluble fraction of the Papilio
retina with UV light, all-trans-or13-cis-retinol to the
fraction, and analyzed the fluorescence of the proteins with
native PAGE. As shown in Fig. 3, both all-trans-and13-cis-
retinols restored the fluorescence of Papilio RBP. This result
indicated that the protein could bind the exogenously added
retinols in vitro, irrespective of their isomeric form.
We next performed spectrophotometry and spectro-
fluorometry of the Papilio RBP. Besides the principal peak
at 280 nm, corresponding to the absorption of the apopro-
tein, the absorbance spectrum (Fig. 4A) of the Papilio RBP
has a secondary peak at 330 nm, corresponding to the
absorption of 3-hydroxyretinol. The rather broad emission
spectrum elicited by 330-nm light (Fig. 4B), peaks at
480 nm, and is very similar to that of free 3-hydroxyretinol
[24]. This indicates that the binding of the apoprotein has
little influence on the fluorescence profile of 3-hydroxy-
retinol. The excitation spectrum (Fig. 4B), measured at an
emission wavelength of 480 nm, shows two maxima at
332 nm and at 280 nm. The principal peak at 332 nm
corresponds to the absorbance spectrum of the ligand,
3-hydroxyretinol. The distinct secondary peak at 280 nm
indicates energy transfer from the apoprotein to the ligand.
Primary structure of
Papilio
RBP
To determine the primary structure of the identified Papilio
RBP, we first analyzed the amino acid sequences of lysyl-
endopeptidase-digested fragments of purified protein. Based
on the sequence results, we designed oligonucleotide primers
and carried out RT-PCR to amplify fragments of cDNA
encoding the protein, and determined its nucleotide
sequence. Subsequently, we performed 3¢-and5¢-RACE
protocols, and obtained the complete nucleotide sequence of
the full-length cDNA encoding the protein (Fig. 5). The
cDNA is approximately 1 kb in length, and contains an
open reading frame of 708 bases encoding 235 amino acid
residues. A stop codon (TAA at nucleotides )9to)7)
precedes the ATG at nucleotides 1–3, suggesting that the
coding region begins at this ATG. A polyadenylation signal,
AATAAA, exists 16 bases upstream from the start of the
poly(A)
+
tail.
Fig. 2. HPLC analysis of the intrinsic ligand of Papilio RBP. The lig-
ands were extracted from Papilio RBP purified from the soluble
fraction of the dark-adapted retina. Extraction and analysis were
carried out under dim red light as follows. Purified protein was first
mixedwith2
M
hydroxylamine and cold 90% methanol to convert
aldehydes, if any, to oximes. Retinoids and oximes were then extracted
with dichloromethane and n-hexane, and separated by normal phase
HPLC. Eluent was monitored for absorbance at 340 nm. Each isomer
of 3-hyroxyretinaloxime and 3-hydroxyretinol was identified by its
retention time compared to that of the standard compound. Purified
Papilio RBP exclusively binds 3-hydroxyretinol. No isomers of
3-hydroxyretinal (detectable as 3-hydroxyretinaloxime, if present) were
detected. AT, all-trans 3-hydroxyretinol; 13, 13-cis 3-hydroxyretinol;
11, 11-cis 3-hydroxyretinol.
Fig. 3. Binding of exogenous ligands to Papilio RBP. Fluorescence of
native PAGE (left) and Coomassie Brilliant Blue (CBB) stained gel
(right). Soluble proteins from the dark-adapted Papilio retina (lanes 1
and 1¢) were irradiated with intense UV-light to degrade intrinsic
ligand (lanes 2 and 2¢). To the UV-irradiated samples, all-trans (lanes 3
and 3¢)or13-cis (lanes 4 and 4¢) retinol was added, followed by
20-min incubation on ice. In this experiment, retinol was used for
3-hydroxyretinol.
2440 M. Wakakuwa et al. (Eur. J. Biochem. 270)FEBS 2003


























