
1
Lecture Date: February 11th, 2008
Nuclear Magnetic Resonance 1
Nuclear Magnetic Resonance
Reading for NMR:
– Chapter 19 of Skoog, et al.
– Handout: “What SSNMR can offer to organic chemists”
Nuclear Magnetic Resonance (NMR)
– Nuclear spin transitions, in the 5-900 MHz range
– Magnetic resonance imaging (MRI)

2
The Electromagnetic Spectrum
NMR, MRI
EPR/ESR
What is NMR?
NMR is an experiment in which the resonance
frequencies of nuclear magnetic systems are
investigated.
NMR always employs some form of magnetic field
(usually a strong externally applied field B0)
NMR is a form of both absorption and emission
spectroscopy, in which resonant radiation is absorbed by
an ensemble of nuclei in a sample, a process causing
detectable emissions via a magnetically induced
electromotive force.
A. Abragam, The Principles of Nuclear Magnetism, 1961, Oxford: Clarendon Press.

3
Things that can be learned from NMR data…
Covalent chemical structure (“2D structure”)
– Which atoms/functional groups are present in a molecule
– How the atoms are connected (covalently bonded)
3D Structure
– Conformation
– Stereochemistry
Molecular motion
Chemical dynamics and exchange
Diffusion rate
3D Distribution of NMR spins in a medium – an image!
– (Better known as MRI)
Plus many more things of interest to chemists…
History of NMR
1920-1930: physics begins to grasp the
concepts of electron and nuclear spin
1936: C. J. Gorter (Netherlands) attempts to
study 1H and 7Li NMR with a resonance
method, but fails because of relaxation
1945-6: E. M. Purcell (Harvard) and F. Bloch
(Stanford) observe 1H NMR in 1 kg of parafin at
30 MHz and in water at 8 MHz, respectively
1952: Nobel Prize in Physics to Purcell and
Bloch
1957: P. C. Lauterbur and Holm independently
record 13C spectra
1991: Nobel Prize in Chemistry to R. R. Ernst
(ETH) for FT and 2D NMR
2002: Nobel Prize in Chemistry to K. Wuthrich
2003: Nobel Prize in Medicine to P. C.
Lauterbur and P. Mansfield for MRI
P. C. Lauterbur F. Bloch
E. M. Purcell R. R. Ernst
Photographs from www.nobelprize.org
4
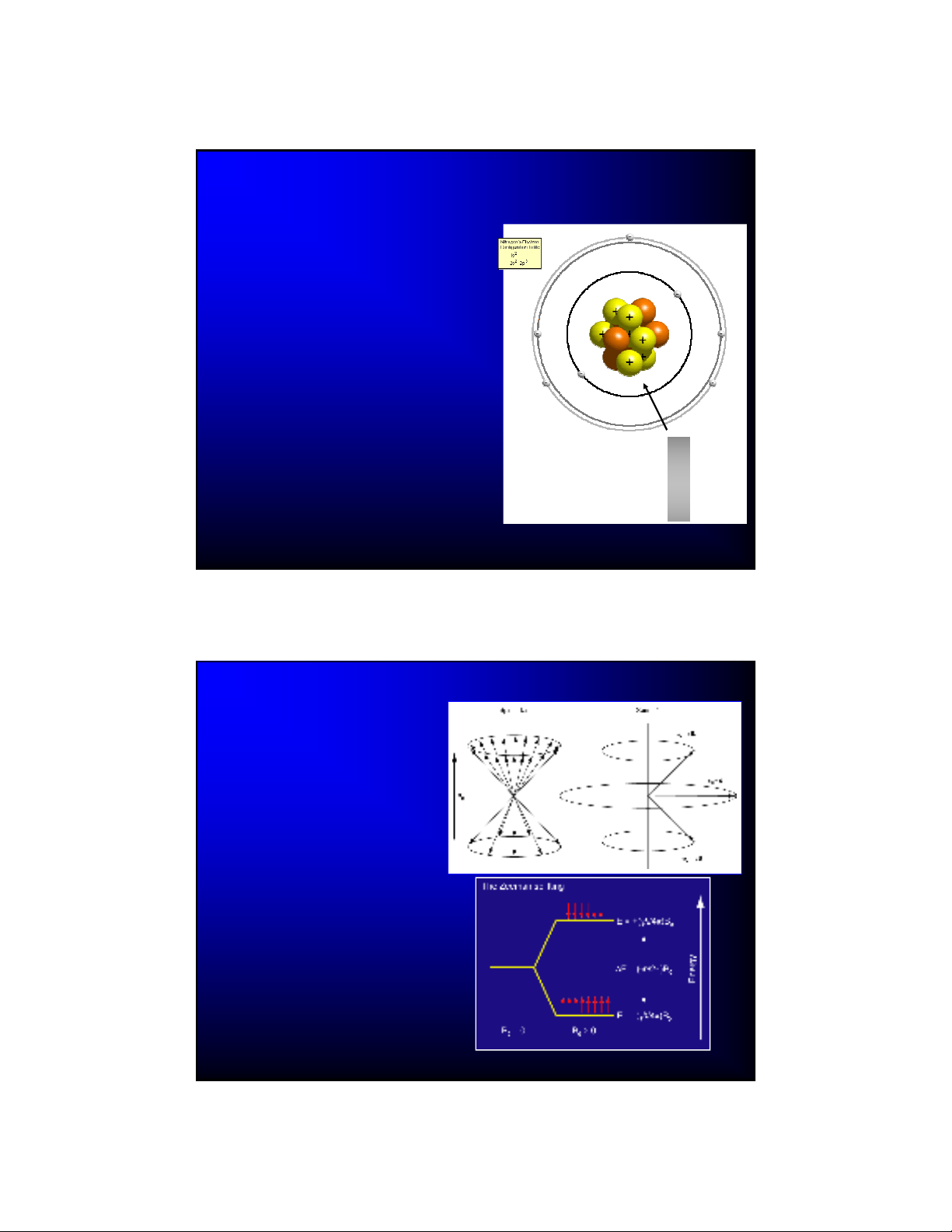
Nuclear Magnetism
A nuclear electromagnet is
created by the nucleons
(protons and neutrons) inside
the atomic nucleus.
This little electromagnet has a
magnetic moment (J T-1)
– The magnetic moment is
proportional to the current flow
through the “nuclear loop”
The nucleus looks like a dipole
to a distant charge center
N
S
From
http://education.jlab.org
Basic NMR Theory
In a strong applied magnetic field
(B0), certain atomic nuclei will
align or oppose this field.
This alignment is caused by the
magnetic moments of the nuclei,
which themselves are caused by
the internal structure of the
nucleus. Two nuclear properties
stand out:
– Spin (1/2 for 1H, 13C, etc…)
– Gyromagnetic ratio
An excess of alignments is found
in the lower energy state
(determined by a Boltzmann
distribution).
At room temperature, this excess
is very small, typically only 1 part
per trillion!
5
Nuclear Spin
In a classical sense the bulk nuclear
magnetization is observed to
“precess” at the Larmor frequency
(usually several hundred MHz):
The constant
is the magnetogyric
ratio.
2
0
0
B
00 B
angular (rad/s) linear (Hz, cycles/s)
B0
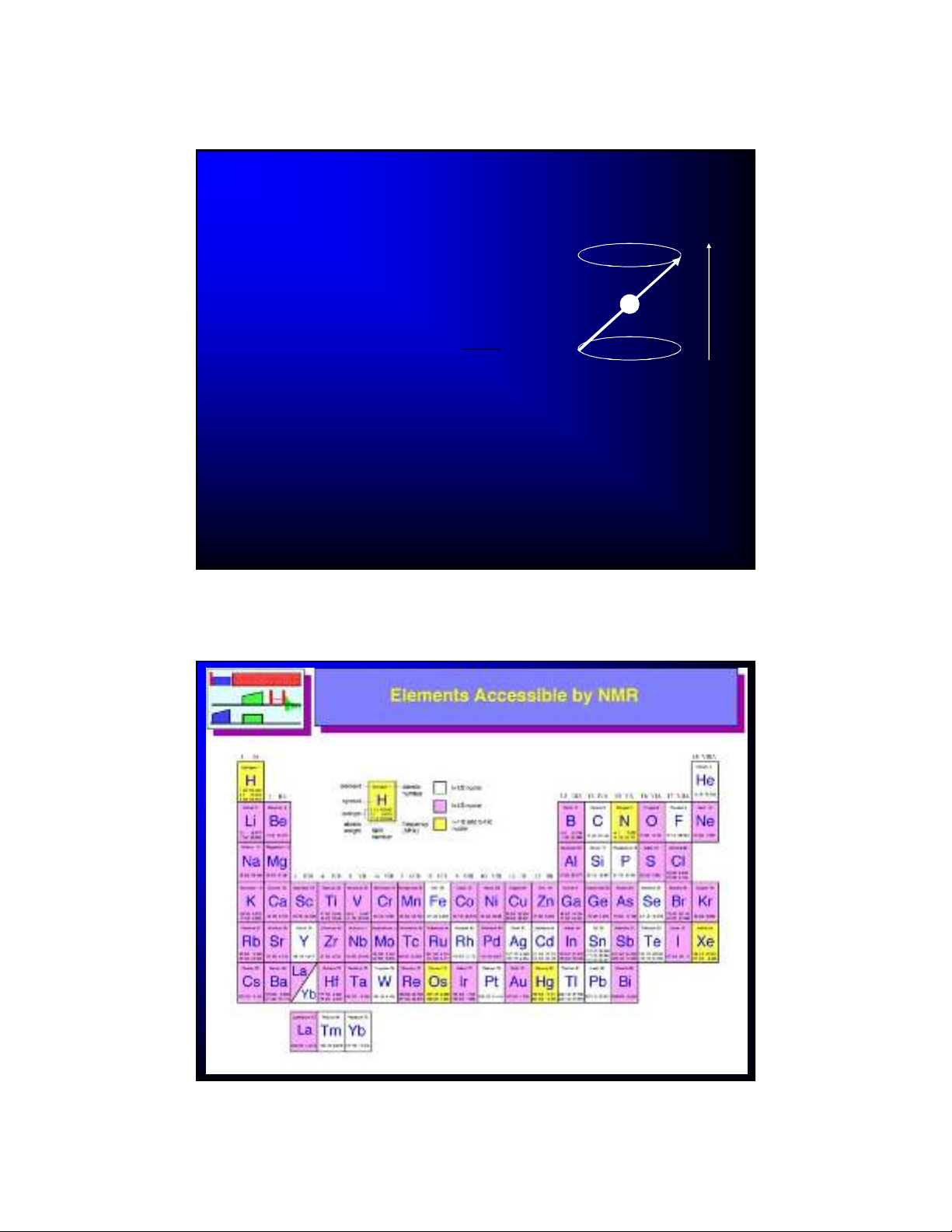
Elements Accessible by NMR
Figure from UCSB MRL website
White = only spin ½
Pink = spin 1 or greater (quadrupolar)
Yellow = spin ½ or greater

![Công nghệ sản xuất Phenol và Phenolfomandehyde [Chuẩn Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2014/20141010/ntchung8894/135x160/1718133_249.jpg)




![Đồ án hóa công công phần chưng luyện [2024]](https://cdn.tailieu.vn/images/document/thumbnail/2013/20130407/ntchung8894/135x160/8711365386768.jpg)
![Quá trình hydrocracking là gì? [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2012/20120922/bibocumi6/135x160/1247748_167.jpg)


















