TRƯỜNG ĐẠI HỌC BÁCH KHOA ĐÀNẴNG
KHOA HOÁ
PHÂN TÍCH POLYME
(POLYMER ANALYSIS)
TS. ĐoànThịThu Loan
♣♣♣
Is a branch of polymer science dealing with analysis and characterisation of
polymers.
The complication of macromolecular chains, the dispersion in molecular
weight, tacticity, crystallinity, orientation, composition of polymers etc. and
complex morphological systems
⇒analysis of polymer ≠the small organic materials
⇒Focus on viscoelasticproperties, dynamic mechanical testing.
Polymer analysis
•Instronmechanical tester
•Vickerhardness tester
•DMA
•Melt flow indexer
•Torsions Rheometer
•
•-AFM, SEM
•-FT-IR
•-Pull-out test
Instruments
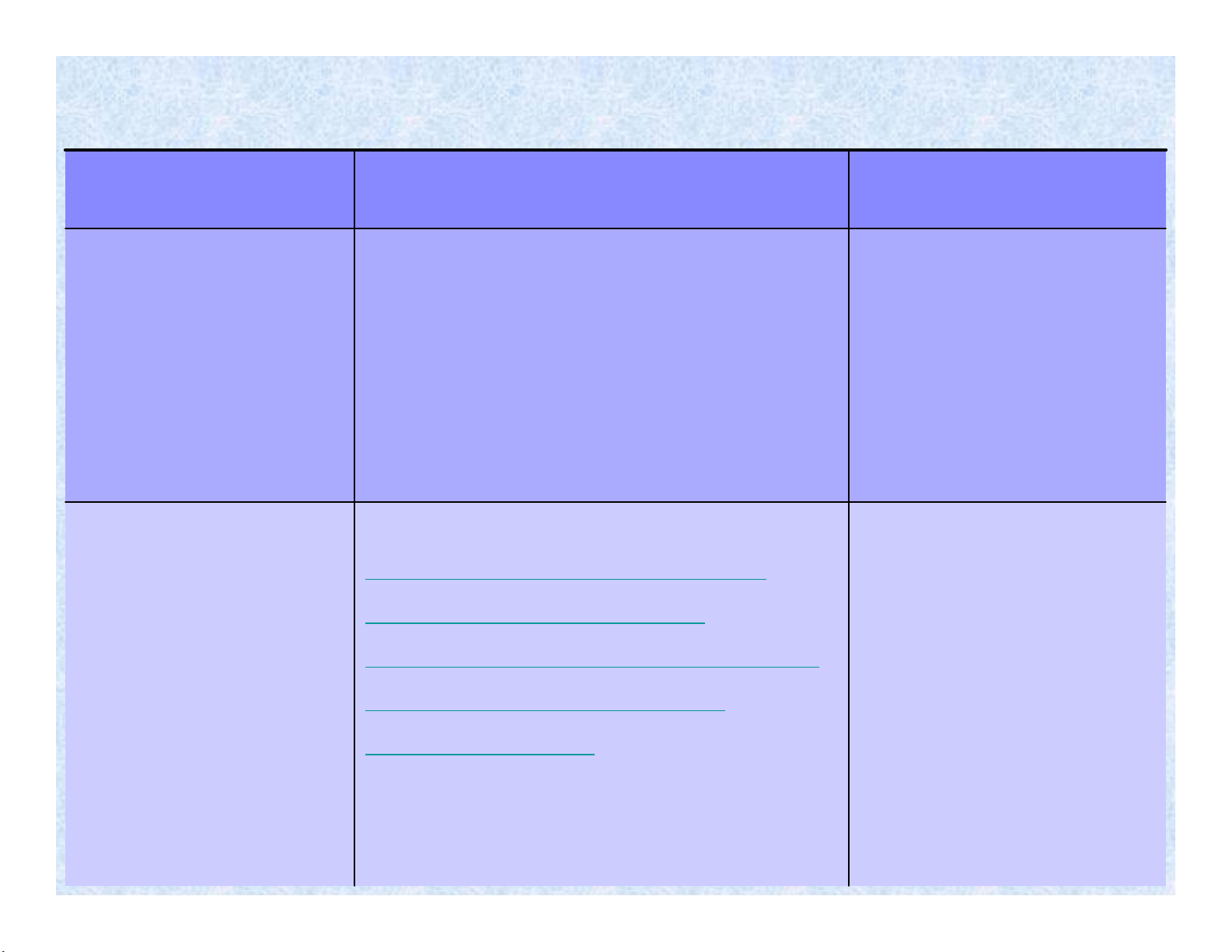
•FT-IR
•IR-microscope
•GPC ( size exclusion chromatography
SEC)
•-Viscosimetry
•-X-ray (WAXS and SAXS)
•-EM, SEM, TEM, AFM
•-Dynamic and static methods for contact
angle measurements.
-Tensile, flexural, impact,
compression, hardness tests,
-Rheologicaland viscoelastic
properties, stiffness and
modulus, surface tension,
permeation and diffusion in
polymers, adhesion tests,
density
-Surface roughness,
-Chemical
composition,
-Interface
characetrisation
-Molecular weight determination,
-Microstructuralcharacterisation and
compositional analysis,
-Crystallinity,
-Investigation of polymer morphology,
particle size,
-Contact angle and wettability
measurements
Mechanical and Physical
Properties
Surface
Characterisation
Chemical, Molecular and Structural
Characterisation
Methods of polymer analysis
•GC
•pH meter
•HPLC
•Karl-Fischer titration
•Thermogravimetricanalyser (TGA)
•TGA-FTIR coupled technique
•Differential scanning calorimetry(DSC)
•Modulated differential scanning
calorimetry(ADSC)
•Dynamic thermomechanicalanalyser
(DMTA)
•Dielectric relaxation
Instruments
Inolabconductivity
meter
•
Purity and molecular
weight of small
molecules, water content
in organic solvents,
surface tension
measurement, pH
-Melting point, glass transition
temperature, free rotation temperature,
-Degradation and stability behaviour of
polymers
Conductivity, electric
current in solution,
light emitting and
electromagnetic
properties
Miscellaneous (hontap)Thermal Behaviour
Electrical and Optical
Properties
Methods of polymer analysis
-For quality control
-For predicting service performance
-To generate design data
-To investigate failures
Purpose of polymer analysis
Essential to identify the purpose of testing, because the requirements for each
of the purposes are different.
-Precision
-Reproducibility
-Rapidity
Balance of these attributes,
according to the purpose of
the test
-Complexity
-Automated test
-Nondestructive test
-Cost




![Bài giảng Tính chất cơ lý của vật liệu polymer [chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2016/20160304/phamvanphat1891995/135x160/5961457069939.jpg)








![Bài giảng Chế biến khoáng sản vô cơ [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251025/thanhvan173002/135x160/21521761538638.jpg)








