1. The responsibilities of Investigator to ensure the safety of
patients, as mentioned in ICH-GCP
2. Circular 6586/BYT-K2ĐT issued date 02 Oct 2012
(Administration of Science Technology and Training,
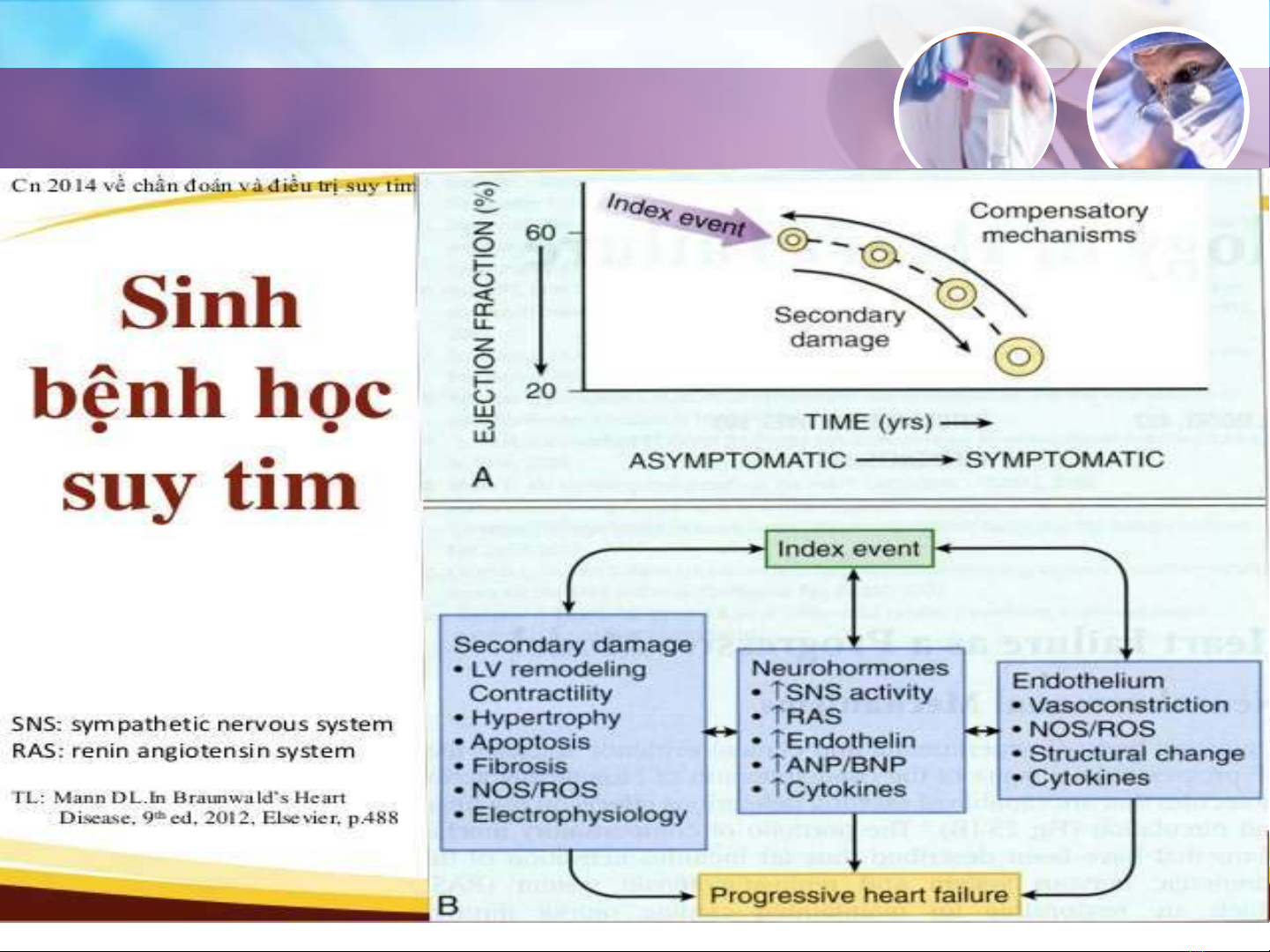
Sinh lý bệnh của Suy Tim
(Administration of Science Technology and Training,
Ministry of Health): Guidance about recording, handling
and reporting Serious Adverse Events (SAEs) in clinical
trials in Vietnam
3. Regulations of the Drug Administration of Vietnam for
current pharmaceutical products
4. Safety reporting process of Sanofi used for all countries