1
MINISTRY OF EDUCATION VIETNAM ACADEMY
AND TRAINING OF SCIENCE AND TECHNOLOGY
GRADUATE UNIVERSITY OF SCIENCE AND TECHNOLOGY
-----------------------------
----------------
Kieu Ngoc Minh
FABRICATION OF FLOWER-LIKE, DENDRITE-LIKE
NANOSTRUCTURES OF GOLD AND SILVER ON SILICON FOR
USE IN THE IDENTIFICATION OF SOME ORGANIC
MOLECULES BY SURFACE ENHANCED RAMAN SCATTERING
Major: Electronic material
Code: 9 44 01 23
SUMMARY OF MATERIAL SCIENCE DOCTORAL THESIS
Ha Noi – 2020
2
This thesis was accomplished in: Graduated University of Science and
Technology – Vietnam Academy of Science and Technology.
Supervisor: 1. Prof. Dr. Dao Tran Cao
2. Dr. Cao Tuan Anh
Peer reviewer 1:
Peer reviewer 2:
Peer reviewer 3:
This thesis will be defended in:
The dissertation will be defended in front of the Institute of Doctoral
Dissertation Assessment Council, taking place at the Academy of Science
and Technology - Vietnam Academy of Science and Technology
at ... hour .... ', day ... month ... year 2020
This thesis will be stored in:
- Library of Graduated University of Science and Technology
- Vietnam National Library

1
Prologue
SERS (surface-enhanced Raman scattering) is a modern analytical
technique that is being strongly researched in the world and Vietnam to
detection trace (ppm-ppb range) of many different molecules, especially organic
and biological molecules. In SERS technique, the most important is the SERS
substrate. The SERS substrate is a rugged continuous or discontinuous precious
metal (silver or gold) at the nano-scale. When analyte molecules are added to
this surface, the signal of Raman scattering of the analyte molecule is greatly
enhanced. Thus, it can be said that SERS substrate is the device that amplifies
Raman scattering signal of the analyte molecule.
In Vietnam, there are some researches on the fabrication of Ag, Au
precious metal nanostructures and using as SERS substrates. However, the
researches mainly focus fabricate nanoparticle structures and so far, fabrication
of silver nano-dendrites (AgNDs), silver nano-flowers (AgNFs) and gold nano-
flowers (AuNFs ) very few, especially the statements on fabrication of these
structures on silicon. For the purpose of studying and researching AgNDs,
AgNFs and AuNFs materials on silicon as well as the properties and
applications of this material, I chose the title of the thesis is “Fabrication of
flowers-like, dendrites-like nanostructure of silver and gold on silicon for
using in detection some organic molecules by surface enhanced Raman
scattering”
In this thesis, we research and fabricate AgNDs, AgNFs, AuNFs
structures on silicon by chemical deposition and electrochemical deposition
method for the main purpose of using as SERS substrate. To this target, we have
studied the morphology, structure and some properties of the nanostructures
produced. Then, we use the nanostructures mentioned above as SERS substrates
to detect traces of some toxic organic molecules, to test their effectiveness as a
SERS substrate.
The scientific significance of the thesis
The AgNDs, AgNFs, AuNFs structures on silicon have been
successfully fabricated by two methods of chemical deposition and
electrochemical deposition with the main purpose for using as SERS substrate.
The influence of fabrication parameters on morphology and structure of
AgNDs, AgNFs, AuNFs was studied in orderly.
The mechanism of formation of the above structures has been studied.
Đã nghiên cứu sử dụng các cấu trúc nano nói trên như là đế SERS để
phát hiện một số phân tử hữu cơ độc hại ở nồng độ thấp.
These nanostructures have been used as SERS substrates to detect some
toxic organic molecules in low concentrations.

2
The thesis includes 4 chapters as follows: This thesis includes of 125
pages (excluding references) with the following layout:
Introduction: Presenting the reasons for choosing topic, methods, purposes of
researching.
Chapter 1: Overview of surface enhanced Raman scattering.
Chapter 2: Methods to fabricate and investigate SERS substrates.
Chapter 3: Fabrication and investigation of silver and gold nanostructures on
silicon.
Chapter 4: Using gold, silver nanostructures like flowers and dendrites as
SERS substrates to detect traces of some organic molecules.
Conclusion: Presenting the conclusions drawn from the research results.
Chapter 1
Overview of surface enhanced Raman scattering
1.1. Raman scattering
Raman scattering is inelastic scattering of a photon with material, discovered by
Raman and Krishnan in 1928. The frequency of scattering light changes
compared to incident light frequency. This amount of change is exactly equal to
the oscillation frequency of the matter molecule and does not depend on the
frequency of the incident light. So, Raman scattering is specific to each
molecule. Raman scattering include of two types: Stockes Raman and anti-
Stockes Raman. It should be noted that the intensity of the Raman effect is
usually very low (about 10-8 - one hundred million incident photons then one
photon is Raman scattering).
1.2. Surface enhanced Raman scattering.
Surface enhancement Raman scattering is a phenomenon that when light fly to
the analyte molecule adsorbed on the surface of a rugged metal nanostructure,
the intensity of the Raman scattering is greatly increased. The metal nanosurface
is called SERS substrate.
There are two enhancement mechanisms for SERS, which are electromagnetic
enhancement mechanism and chemical enhancement mechanism. In which,
electromagnetic enhancement mechanism is main contributor.
1.2.1. Electromagnetic enhancement mechanism
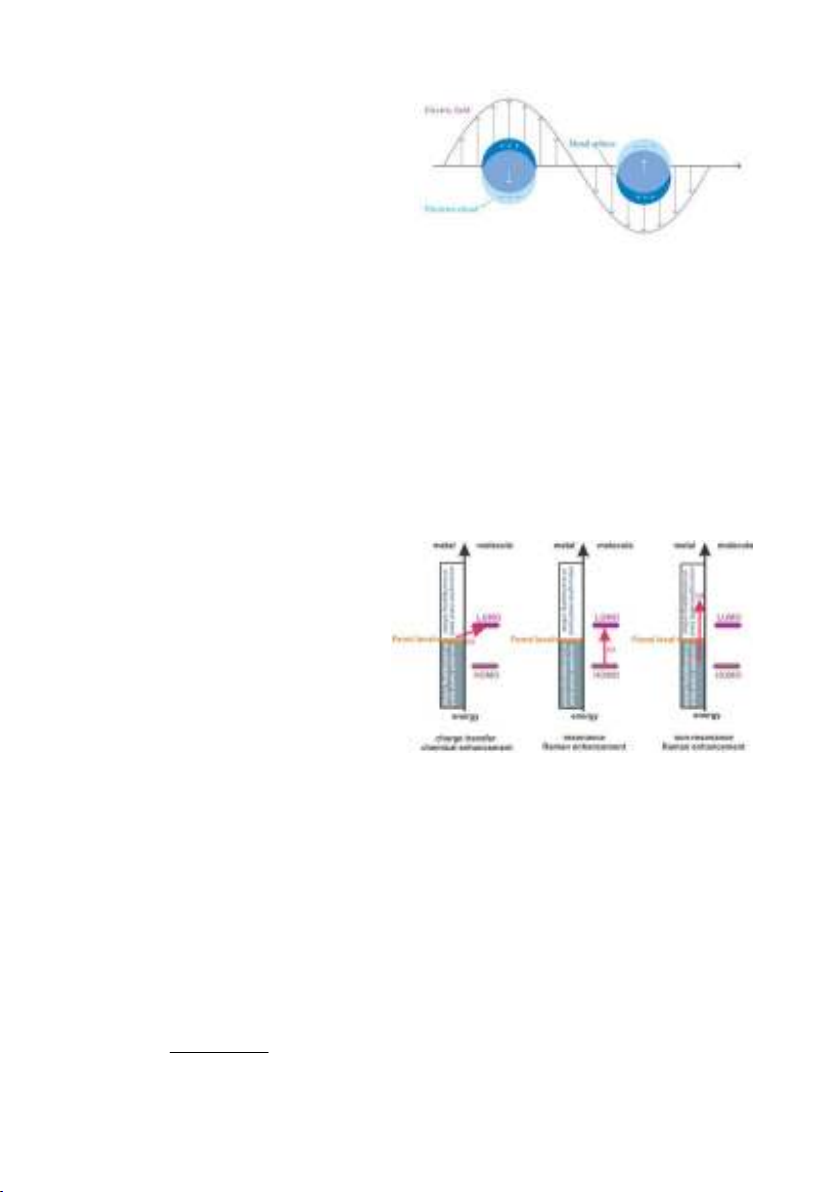
Surface localized plasmon resonance (LSPR) occurs when the surface plasmon
is confined to a nanostruc-ture that Size is smaller than the wavelength of light.
From the Fig 1.5, it can see that the electric field of the incident light is an
oscillating electric field. In the first half of the cycle, the incident electric field is
directed upwards, which has the effect of causing the conduction electrons to
move downwards in metal nanoparticles.
3
Thus, the top part of the metal
nanoparticles will be positively
charge, resulting the metal
nanoparticles becoming an dipole. In
the second half of the cycle, the
electric field of the incident light
changes direction, the dipole also
changes direction. As a result, the
dipole also oscillates with the
frequency of the incident light. The
vibrating dipole produces an
electromagnetic field (new light
source).
Fig 1.5. Schematic illustration of surface
localized plasmon resonance (LSPR)
with free conducting electrons in metal
nanoparticles that are oriented by
oscillation due to strong connection with
incident light.
If the new electromagnetic field vibrates with the oscillation frequency of the
incident light, then we have a resonance. The result, the incident light field is
enhanced by E
2
times while the scattering field is also enhanced by E
2
times, the
total field is enhanced by E
4
times.
1.2.2. Chemical enhancement mechanism
The presence of chemical
mechanism with Raman
scattering was observed when
plasmonic metals are not used.
Studies of non-electromagnetic
enhancement mechanisms have
shown that resonancing between
incident light and metal
nanostructures can induce charge
transfer between analyte
molecules and metal.
Fig 1.6. Three different types of chemical
enhancement mechanisms in SERS.
Charge transmission occurs, the metals and molecules of the analyte must be in
direct contact with each other. In other words, charge transmission occurs only
when the metals and molecules are close enough that the wave functions
overlap. The exact mechanism of charge transfer has not been fully understood
until now.
1.3. SERS enhancement factor
The SERS enhancement factor used in the thesis is the SERS substrate
enhancement factor (SSEF) and is calculated by the following formula:
SERS Normal
Normal SERS
I N
SSEF I N





![Luận văn Thạc sĩ: Tổng hợp và đánh giá hoạt tính chống ung thư của hợp phần lai tetrahydro-beta-carboline và imidazo[1,5-a]pyridine](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250816/vijiraiya/135x160/26811755333398.jpg)








![Đề án Thạc sĩ: Tổ chức hoạt động văn hóa cho sinh viên Trường Cao đẳng Du lịch Hà Nội [Chuẩn nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251202/kimphuong1001/135x160/91661764646353.jpg)











