
The sequentiallity of nucleosomes in the 30 nm
chromatin fibre
Dontcho Z. Staynov
1
and Yana G. Proykova
2
1 Imperial College London, National Heart and Lung Institute, UK
2 School of Earth and Environmental Sciences, University of Portsmouth, UK
The DNA is packed on several levels as chromatin in
the eukaryotic nucleus. The first level of packing,
the highly conserved nucleosome, allows transcrip-
tion, after remodelling and ⁄or histone modifications ⁄
replacements. The nucleosome core particles have been
reconstituted and crystallized and their structure solved
in detail at 1.9 A
˚resolution [1–3]. The second level of
packing is the transcriptionally dormant 30 nm chro-
matin fibre. Understanding its structure, as well as the
processes that determine its folding and unfolding, is a
prerequisite for studying the epigenetic mechanism,
which leads to poised-for-transcription or dormant
chromatin [4]. The fibre consists of the entire chroma-
tin of the nucleated avian erythrocytes and comprises
approximately 85% of the chromatin in other cell
types [5].
The structure of chicken erythrocyte chromatin is
the most widely studied in the whole nucleus, as well
as in solution. Using small angle X-ray and neutron
scattering, it has been shown that all the high mole-
cular weight material that diffuses out of the nuclei
after micrococcal nuclease (MNase) digestion is in the
30 nm fibre conformation. It consists of a regular helix
with a diameter of approximately 33 nm and a variable
mass per unit length, which approaches 0.6 nucleo-
somesÆnm
)1
with an 11 nm pitch at 80 mmsalt concen-
trations. This implies that there are seven nucleosomes
per helical turn with their flat surfaces almost parallel
to the fibre axis [6–11]. The unusually small cross-
sectional radius of gyration (9.5 nm at 80 mmsalt)
suggests a very compact structure with close nucleo-
some–nucleosome contacts.
There are several basic models for the structure of
the fibre that were proposed in the late 1970s and early
1980s, and some variants have been published subse-
quently [4,5,12]. They all comprise regular helices of
more or less seven nucleosomes per turn and thus
approximately satisfy the results obtained by small
angle X-ray and neutron scattering and low resolution
electron microscopy with respect to the packing of
Keywords
30 nm fibre; chromatin structure;
nucleosome
Correspondence
D. Z. Staynov, Imperial College London,
National Heart and Lung Institute, Guy
Scadding Building, Dovehouse Street,
London SW3 6LY, UK
Tel: +44 207 6223644
E-mail: d.staynov@imperial.ac.uk
(Received 29 March 2008, revised 20 May
2008, accepted 23 May 2008)
doi:10.1111/j.1742-4658.2008.06522.x
The folding of eukaryotic DNA into the 30 nm fibre comprises the first
level of transcriptionally dormant chromatin. Understanding its structure
and the processes of its folding and unfolding is a prerequisite for under-
standing the epigenetic regulation in cell differentiation. Although the
shape of the fibre and its dimensions and mass per unit length have been
described, the path of the internucleosomal linker DNA and the sequential-
lity of the nucleosomes in the fibre are poorly understood. In the present
study, we have chemically crosslinked adjacent nucleosomes along the
helix of chicken erythrocyte oligonucleosome fibres, digested the inter-
nucleosomal linker DNA and then examined the digestion products by
sucrose gradient sedimentation. We found that the digestion products con-
tain considerable amounts of mononucleosomes but less dinucleosomes,
which suggests that there are end-discontinuities in the fibres. This can be
explained by a nonsequential arrangement of the nucleosomes along the
fibre helix.
Abbreviations
as, acid soluble; DSP, dithiobis-(succinimidyl propionate); EDC, 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide; MNase, micrococcal nuclease.
FEBS Journal 275 (2008) 3761–3771 ª2008 The Authors Journal compilation ª2008 FEBS 3761

nucleosomes in the fibre. However, they were proposed
before the crystal structure of the nucleosome was
solved and do not take into account the topological
constraints imposed on the relationship with respect to
nucleosome orientation and tilt versus chromatin
repeat length. Thus, they differ with respect to the ori-
entation of the nucleosomes and the path of the linker
DNA within the fibre.
High definition structures have not been achieved
because the native fibres comprise a mixture of differ-
ent repeat lengths and could not be crystallized. To
avoid this problem, several studies recently reconsti-
tuted oligonucleosome arrays on a nucleosome by
positioning DNA sequence-repeats differing by multi-
ples of 10 or 11 bp [13–16]. Dorigo et al. [14] used cys-
teine substituted recombinant core histones with or
without linker histone. The electron micrographs of
their reconstitutes show flat ribbons with approxi-
mately five instead of seven nucleosomes per 11 nm,
which do not fold into helical fibres, although they
refer to them as two-start helices. To study nucleosome
sequentiallity in their reconstitutes, Dorigo et al. [14]
crosslinked the samples and digested the linker DNA
with a restriction enzyme. The resultant oligonucleo-
somes migrate in a ‘native’ gel as a ladder with dimers
up to half the size of the original material and support
a two-start helix. In a subsequent study by Schalch
et al. [16], a reconstituted tetranucleosome with a
20 bp linker DNA was crystallized. It was speculated
that this construct allows a two-stranded helix with
close nucleosome contacts in which the flat surfaces of
the nucleosomes are perpendicular instead of parallel
to the fibre axis.
Very different results were obtained by Robinson
et al. [15] using native chicken erythrocyte core histones
and H5 linker histone. They observed two helical
arrays: one for repeat lengths below 210 bp with a
diameter of 33 nm and another for repeat lengths above
210 bp with a diameter of 45 nm and with the flat
surfaces of the nucleosomes close to parallel to the fibre
axis. The overall shapes of their reconstitutes are very
similar to the fibres observed in ‘native’ chromatin.
Most striking are the two very different structures
presented by the two groups for the 177 bp as well as
the 207 and 208 bp repeat lengths, which differ by the
presence ⁄absence of the linker histone. Apparently,
the reconstitutes of the two groups cannot represent
the same structure and additional evidence is needed.
Both groups have discussed their results with respect
to discriminating between single-start (sequentially
arranged nucleosomes) and two-start nonsequential
helices. Other possible nonsequential helices were
ignored. Neither group considered the very informa-
tive results obtained by DNase I digestion of native
chromatin, which produces ‘dinucleosome repeat’ pat-
terns. Such patterns in which the even multiples are
strong can be produced only if the adjacent nucleo-
somes are digested at alternating sites and, thus, the
odd multiple fragments are attenuated and the even
multiple fragments dominate the pattern. These results
unambiguously show that there is a common structure
of the fibre in which the consecutive nucleosomes in
samples of several different repeat lengths have alter-
nating orientations, as extensively discussed elsewhere
[5,12,17,18].
The question of the sequentiallity of the nucleo-
somes in the fibre is essential. Because a variety of
higher order structures might be capable of reconstitu-
tion with a repeat-sequence DNA, a key question is
how do the reconstitutes of the two groups compare
with the fibres obtained from natural chromatin?
In the present study, we examined the sequentiallity of
the nucleosomes in 30 nm fibres, which diffuse out of
chicken erythrocyte nuclei after a mild MNase diges-
tion without further manipulations. We used the ratio-
nale of Dorigo et al. [14], which involved crosslinking
and nuclease digestion. To demarcate the adjacent
nucleosomes along the fibre, we used nonspecific
protein–protein crosslinkers with two different spans:
(a) dithiobis-(succinimidyl propionate) (DSP) (also
known as Lomant’s reagent), a cleavable bifunctional
ester with 1.2 nm span and a noncleavable, contact-site
crosslinker and (b) 1-ethyl-3(3-dimethylaminopropyl)-
carbodiimide (EDC). Subsequently, the samples were
redigested with MNase and fractionated by sedimenta-
tion on sucrose gradients. Instead of obtaining half the
size of the original material, we observed only a slight
decrease of its size and a considerable number of
mononucleosomes. These results do not support the
two-start helix arrangement, but a higher order nonse-
quential arrangement of the nucleosomes in the fibre
with end-defects as described below.
Experimental rationale
It has been shown that, at moderate ionic strength dif-
ferent, crosslinkers can covalently crosslink linker- and
core-histones beyond a single nucleosome and thus are
able to demarcate adjacent nucleosomes along the
helix of the 30 nm fibre [5]. In the present study, we
used internucleosome histone crosslinking and subse-
quent nuclease digestion to distinguish between differ-
ent arrangements of the nucleosomes in the fibre. The
rationale is illustrated in Fig. 1. Three topologically
different arrangements of the nucleosomes along the
fibre have been suggested [5].
Nucleosome sequentiallity in the 30 nm fibre D. Z. Staynov and Y. G. Proykova
3762 FEBS Journal 275 (2008) 3761–3771 ª2008 The Authors Journal compilation ª2008 FEBS
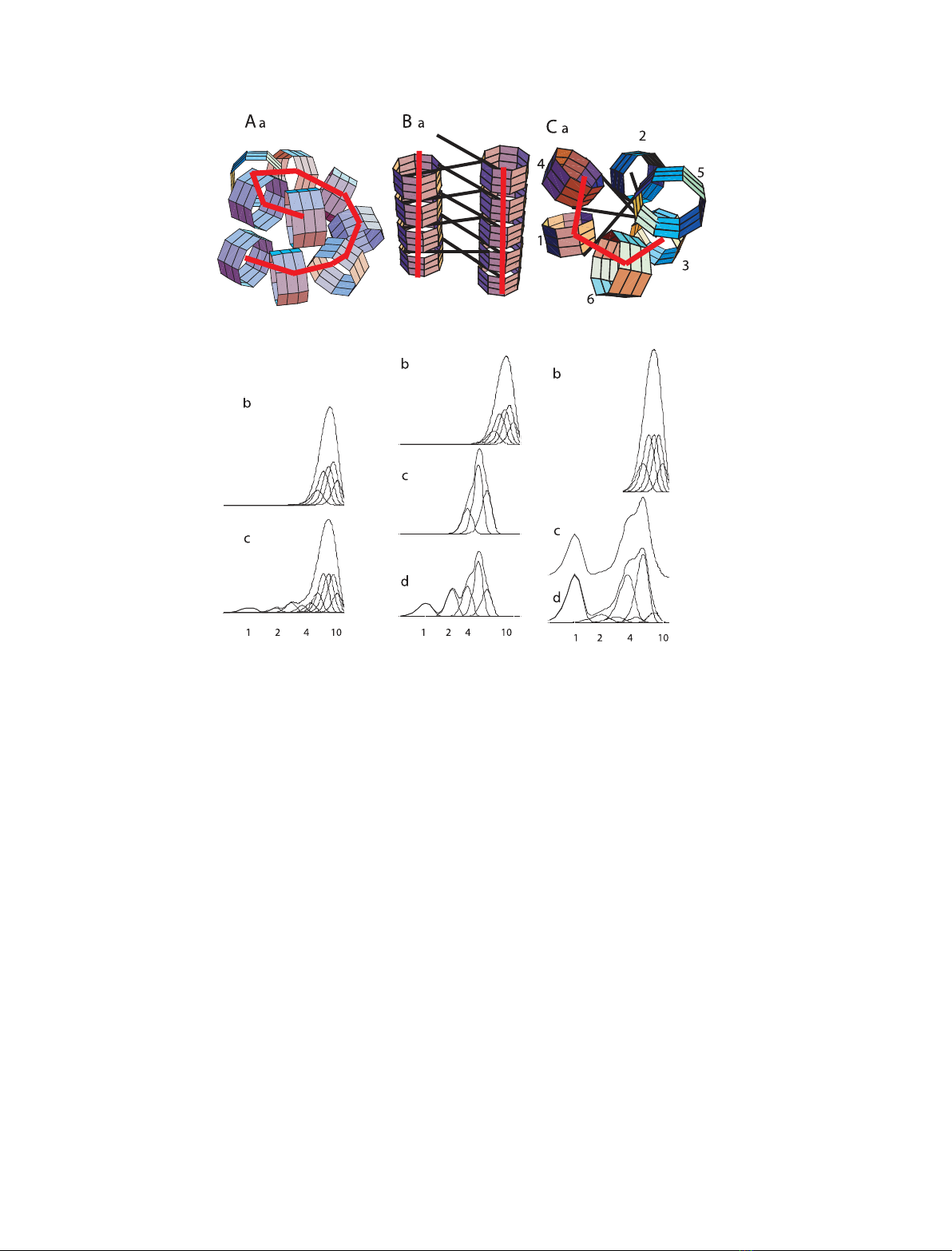
A sequential arrangement
The order of nucleosomes along the fibre helix follows
their order along the DNA. If a 9-mer fragment of
continuous helix of nucleosomes (Fig. 1Aa) is exten-
sively crosslinked, the adjacent nucleosomes will be
crosslinked and the continuity of the linker DNA will
not be required to keep them together. Figure 1Ab
shows the sedimentation profile of an oligonucleosome
sample comprising 7- to 9-mers and half the quantity
of 6- to 10-mers. After 100% crosslinking and nuclease
digestion to mononucleosome size DNA, the sedimen-
tation profile of the sample remains the same. The
80% crosslinked sample will show a decrease of the
average size of the original oligonucleosome sample
and smaller size oligomers will appear (Fig. 1Ac). If
the nucleosomes are interdigitated, as shown by Rob-
inson et al. [15], there will be more internucleosomal
crosslinks, which will stabilize the fibre structure, and
the sedimentation profiles of digestion products will be
intermediate between those shown in Fig. 1Ab,Ac.
A multi-start helix
Nucleosomes are arranged in a multistrand sequence
and are not consecutive. Figure 1Ba illustrates a rib-
bon, which can fold into a two-start helix fibre, with
linkers either parallel or perpendicular to its axis.
Fig. 1. Schematic presentation of different nucleosome arrangements in the 30 nm fibre and the expected sucrose gradient sedimentation
profiles after 100%, 90% or 80% chemical crosslinking of nucleosomes and digestion of the DNA to mononucleosome size of samples con-
sisting of a mixture of hepta-, octa- and nonanucleosomes and half the amount of hexa- and decanucleosomes (6- to 10-mer sample). (A) (a)
Nonamer of a sequential single helix. (b, c) Sedimentation profiles after crosslinking and subsequent digestion of all DNA linkers of the 6- to
10-mer sample: (b) 100% crosslinked and (c) 80% crosslinked. (B) (a) Nonamer in a two start nonsequential (ribbon) arrangement. Numbers
indicate the number of consecutive nucleosomes along the DNA chain. (b–d) Sedimentation profiles of the oligonucleosome sample before
digestion (b) and after digestion of 100% crosslinked (c) and 90% crosslinked sample (d). (C) (a) Hexamer in a single helix nonsequential
arrangement. (b–d) Sedimentation profiles of the 6- to 10-mer sample: (b) original, (c) after digestion of 100% crosslinked and (d) 90% cross-
linked sample. The thick red line demarcates adjacent nucleosomes, expected to be crosslinked. Numbers under the horizontal axes of the
sedimentation profiles denote mono- di-, etc. nucleosomes.
D. Z. Staynov and Y. G. Proykova Nucleosome sequentiallity in the 30 nm fibre
FEBS Journal 275 (2008) 3761–3771 ª2008 The Authors Journal compilation ª2008 FEBS 3763

Digestion of the same mixture of 6- to 10-mers
(Fig. 1Bb) of a 100% crosslinked sample will produce
half the size of the original sample (Fig. 1Bc). If the
crosslinking is not complete, smaller size oligomers will
appear (Fig. 1Bd) but the maximum of the main peak
will be approximately half the size of the original
sample.
Single-stranded helices with nonsequentially arranged
nucleosomes
Due to nucleosomes’ nonsequentiallity, the fibres have
end-defects (not envisaged in either of the structures
shown in Fig. 1A,B); namely, one or two missing end-
nucleosomes, as well as one or two end-nucleosomes
separated from the rest by longer distances. Thus,
these end-nucleosomes are probably non-interacting
with the continuous helix and may not be crosslinked
to the rest. One such arrangement, the (–3,5) arrange-
ment, is shown in Fig. 1Ca [18]. Thus, nuclease diges-
tion will shorten the size of the original sample on
average by three nucleosomes and will produce a frac-
tion of 3 ⁄nmononucleosomes, where nis the average
number of nucleosomes per fragment. The expected
sedimentation profiles of the same 6- to 10-mer sam-
ple, before and after nuclease digestion, are shown in
Fig. 1Cb–d. Because the closely interacting nucleo-
somes are always an even number (Fig. 2), digestion
will produce a mononucleosome fraction and a mix-
ture of even multiples. If some of the end-nucleosomes
are crosslinked to the rest via linker–linker or linker–
core histone crosslinks, the mononucleosome fraction
will be less than 3 ⁄nand crosslinking will produce
some odd number oligonucleosomes in the digest.
Thus, the sedimentation profile might not be as clear-
cut as shown in Fig. 1Cc,d, but there would be a
mononucleosome fraction and enriched even-multiples
of oligonucleosomes in the main fraction. Incomplete
(90%) crosslinking will also produce some odd number
oligomers.
As shown in Fig. 1, the differences among the
expected sedimentation profiles of the oligonucleosome
samples after crosslinking and MNase digestion are
expected to be considerable and some incomplete
crosslinking, or cross-chain crosslinking, will not
change their characters.
Figure 2 shows that, in the nonsequential (–3,5)
arrangement, the number of nucleosomes making close
contacts is always even [18]. Thus, there are no close
contacts in the di- and trinucleosomes, whereas only
two nucleosomes are close to each other in tetra- and
pentanucleosomes. In hexanucleosomes, there are four
nucleosomes in close proximity.
Results
Chicken erythrocyte nuclei were digested with MNase
and the material that diffused out of the nuclei was
fractionated by sedimentation through sucrose gradi-
ents. All chromatin samples from dinucleosomes to
high molecular weight material contained indistin-
guishable ratios of linker to core histones (see supple-
mentary Fig. S1).
Chromatin crosslinked with DSP
DSP has been used previously for histone crosslinking
to establish the proximity of different histones inside
the nucleosome or of nucleosomes in the fibre [5,19]. It
is a cleavable crosslinker with two succinimidyl groups,
which react with lysines independently. The maximum
span of crosslinking is 1.2 nm.
A sample of oligonucleosomes, consisting mainly of
tetra-, penta-, hexamers, minor tri- and heptamer com-
ponents with an average number of nucleosomes in the
main peak of 4.9, was extensively crosslinked. It was
digested with MNase for different lengths of time and
agarose gel electrophoresis of the DNA exhibited the
characteristic nucleosome repeat (not shown). After
removal of free crosslinker by dialysis, it was fraction-
ated on sucrose gradients. Figure 3A,B shows that the
di tri
tetra penta
hexa
11
11
1
22
2
2
2
3
3
3
3
44
4
5
5
6
Fig. 2. Schematic presentation of di- to hexanucleosomes in the
(–3,5) arrangement. The thick red lines illustrate closely spaced
nucleosomes. Numbers indicate consecutive nucleosomes along
the DNA chain.
Nucleosome sequentiallity in the 30 nm fibre D. Z. Staynov and Y. G. Proykova
3764 FEBS Journal 275 (2008) 3761–3771 ª2008 The Authors Journal compilation ª2008 FEBS
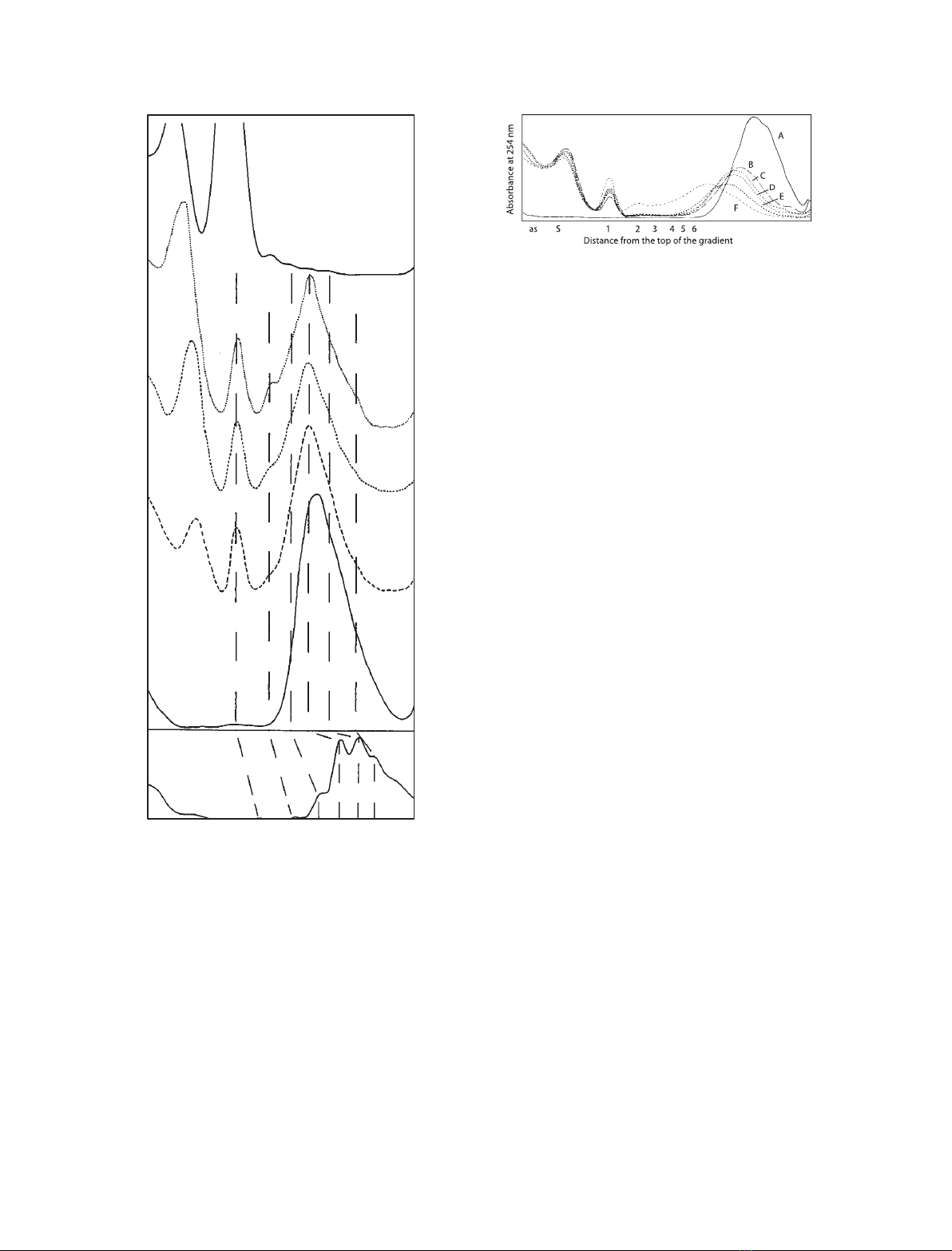
crosslinking resulted in a partial loss of resolution and
a drop in the sedimentation velocities. Digestion with
MNase produced 10% mononucleosomes and as little
as 3.5% dinucleosomes (Fig. 3C). The average number
of nucleosomes per chain in the main peak decreased
to 4.1. One third of the optical density sedimented
slower than the mononucleosome fraction. It com-
prised an acid soluble (as) oligo-nucleotide fraction
(14%) at the top of the gradient, and a well-defined
band, S, of mononucleosome-size naked DNA (17%).
Further MNase digestion (Fig. 3D,E) led to an
increase of band Sby up to 50%, but did not change
the overall result; the proportion of mononucleosomes
increased to 15% and dinucleosomes to 7%. The main
peak was centered at tetranucleosomes (approximately
40%) with the even numbers (2-, 4- and 6-mers)
slightly more pronounced than the odd numbers
(3- and 5-mers). At longer times of digestion, more
than 50% of the sample was converted into the frac-
tion S. Breaking the disulfate bond in the middle of
the crosslinker produced only mononucleosomes and
the band S. DNA gel electrophoresis of the fractions
from Fig. 3D,E showed that approximately 90% of
the DNA in the main peak as well as the mononucleo-
somes and band Sare all in the 140–160 bp size
interval (not shown). The high percentage of mono-
nucleosomes obtained after MNase digestion with only
a small amount of dinucleosomes, as well as the grad-
ual decrease of the number of nucleosomes in the main
peak, indicates that crosslinking is almost complete in
the middle of the fibre, but some of the end-nucleo-
somes are not crosslinked to the rest and therefore
must originate from end-defects in the fibre. In differ-
ent experiments, the mononucleosome fraction was
always in the range 0.9–1.6 per chain (and often higher
than 1.0).
A sample of eight to 12 nucleosomes was cross-
linked, digested with MNase, and further digested with
trypsin for different lengths of time. The sedimentation
profiles are shown in Fig. 4. It is seen that MNase
(Fig. 4B) produces a similar profile as in Fig. 3B, with
prominent fractions as,Sand mononucleosomes, but
that di- to penta-nucleosomes are of negligible
amounts due to the larger size of the starting material.
Absorbance at 254 nm
F
E
D
C
B
A
S123456as
Distance from top of the gradient
Fig. 3. UV absorbance profiles of sucrose gradients of an oligonu-
cleosome sample mainly comprising tetra- penta- and hexamers
and minor tri- and heptamer components. (A) Control (no crosslink-
ing). (B–E) Extensively crosslinked with DSP and digested with
20 unitsÆmL
)1
MNase for 0, 8, 16 and 32 min respectively. (F)
Showing the sample used in (E) but reduced to break the crosslin-
ker. Numerals 1, 2, etc., denote mono-, di-, etc., nucleosomes.
Fig. 4. UV absorbance profiles of sucrose gradients of a sample of
eight to 12 nucleosomes. (A) Control (no crosslinking). (B–F) Exten-
sively crosslinked with DSP, digested with 20 unitsÆmL
)1
MNase
for 20 min and subsequently digested with trypsin for 0, 1.5, 5, 15
and 45 min. Numerals 1, 2, etc., denote mono-, di-, etc., nucleo-
somes.
D. Z. Staynov and Y. G. Proykova Nucleosome sequentiallity in the 30 nm fibre
FEBS Journal 275 (2008) 3761–3771 ª2008 The Authors Journal compilation ª2008 FEBS 3765


























