
MINIREVIEW
Endogenous cardiac glycosides, a new class of steroid hormones
Wilhelm Schoner
Institut fu
¨r Biochemie und Endokrinologie, Justus-Liebig-Universita
¨t Giessen, Germany
The search for endogenous digitalis has led to the isolation of
ouabain as well as several additional cardiotonic steroids of
the cardenolide and bufadienolide type from blood, adre-
nals, and hypothalamus. The concentration of endogenous
ouabain is elevated in blood upon increased Na
+
uptake,
hypoxia, and physical exercise. Changes in blood levels of
ouabain upon physical exercise occur rapidly. Adrenal cor-
tical cells in tissue culture release ouabain upon addition of
angiotensin II and epinephrine, and it is thought that oua-
bain is released from adrenal cortex in vivo. Ouabain levels in
blood are elevated in 50% of Caucasians with low-renin
hypertension. Infusion over several weeks of low concen-
trations of ouabain, but not of digoxin, induces hypertension
in rats. A digoxin-like compound, which has been isolated
from human urine and adrenals, as well various other
endogenous cardiac glycosides may counterbalance their
actions within a regulatory framework of water and salt
metabolism. Marinobufagenin, for instance, whose con-
centration is increased after cardiac infarction, may show
natriuretic properties because it inhibits the a1 isoform of
Na
+
/K
+
-ATPase, the main sodium pump isoform of the
kidney, much better than other sodium pump isoforms. In
analogy to other steroid hormones, cardiotonic steroid
hormones in blood are bound to a specific cardiac glycoside
binding globulin. The discovery of ouabain as a new adrenal
hormone affecting Na
+
metabolism and the development of
the new ouabain antagonist PST 2238 allows for new pos-
sibilities for the therapy of hypertension and congestive heart
failure. This will lead in turn to a better understanding of the
disease on a physiological and endocrinological level and of
the action of ouabain on the cellular level as a signal that is
transduced to the plasma membrane as well as to the cell
nucleus.
Keywords: endogenous digitalis; endogenous ouabain;
ouabain antagonists; cardiotonic steroids; cardiac glyco-
side binding globulin; signal transduction; congestive heart
failure; hypertension.
INTRODUCTION
For more than 200 years, digitalis, a cardiotonic steroid,
and its congeners have been used to treat congestive heart
failure [1]. The beneficial result of this therapy was so
impressive in the first half of the twentieth century that in
1953, Albert Szent-Gyo
¨rgyi postulated the existence of an
endogenous digitalis in mammals [2], thereby reviving a
similar idea that Ringer had published in 1885 [3]. Modern
understanding of digitalis therapy arose 50 years ago, when
in 1953 Schatzmann discovered that cardiotonic steroids are
specific inhibitors of the sodium pump [4] and that the
digitalis receptor is the Na
+
/K
+
-ATPase of plasma mem-
branes [5]. The discovery of the Na
+
/Ca
2+
exchanger in the
late 1960s in mammalian cardiac muscle led to the view that
the inhibition of the sodium pump by cardiotonic steroids
leadstoanincreaseintheconcentrationofintracellular
Ca
2+
as a secondary event, which in turn results in a
positive inotropic effect on cardiac muscle [6]. This model
has been recently refined: it is clear now that the a1 isoform
of the sodium pump is ubiquitously distributed in plasma
membranes of cardiomyocytes but that the a
2
/a
3
isoforms
reside in plasma membrane areas close to the endoplasmic
reticulum. Such ÔplasmerosomesÕalso contain the Na
+
/
Ca
2+
exchanger protein. Inhibition of the a
2
and a
3
isoforms of Na
+
/K
+
-ATPase in such a restricted area leads
to a change in cytosolic Na
+
and, indirectly, Ca
2+
concentrations. This modulates in turn the Ca
2+
content
of the sarcoplasmic reticulum and Ca
2+
signaling, and leads
finally to the positive inotropic effect of cardiac glycosides
[7,8] and an altered gene expression of proteins [9].
The search for an endogenous digitalis-like compound
was aided in the last few decades when it became apparent
that volume-expanded forms of hypertension may lead to
the release of a natriuretic hormone. The Dahl-deWardener-
Blaustein concept [10–12] of a natriuretic hormone proposes
that an enhanced production of endogenous inhibitor(s) of
the sodium pump occurs with the adaptive function
of decreasing the volume of circulating fluid by means of
inhibition of the Na
+
/K
+
-ATPase in renal tubules. The
increased production of endogenous digitalis-like com-
pounds would also contribute to hypertension by means
of inhibition of Na
+
/K
+
-ATPase in cardiovascular tissues
[10–12]. For some time it was not possible to establish that
an endogenous digitalis actually exists. Hamlyn et al. were
the first to demonstrate that the concentration of a
circulating factor in blood plasma inhibiting purified Na
+
/
K
+
-ATPase correlated with the blood pressure of the
donors [13]. This observation paved the way for the
Correspondence to W. Schoner, Institut fu
¨r Biochemie und Endokri-
nologie, Fachbereich Veterina
¨rmedizin, Justus-Liebig-Universita
¨t
Giessen, Frankfurter Str. 100, D-35392 Giessen, Germany.
Tel.: + 49 641 99 38171; Fax: + 49 641 99 38179,
E-mail: wilhelm.schoner@vetmed.uni-giessen.de
Abbreviations: FAB-MS, fast atom bombardment mass spectrometry;
ACE, angiotensin converting enzyme.
(Received 15 October 2001, revised 25 February 2002,
accepted 3 April 2002)
Eur. J. Biochem. 269, 2440–2448 (2002) ÓFEBS 2002 doi:10.1046/j.1432-1033.2002.02911.x
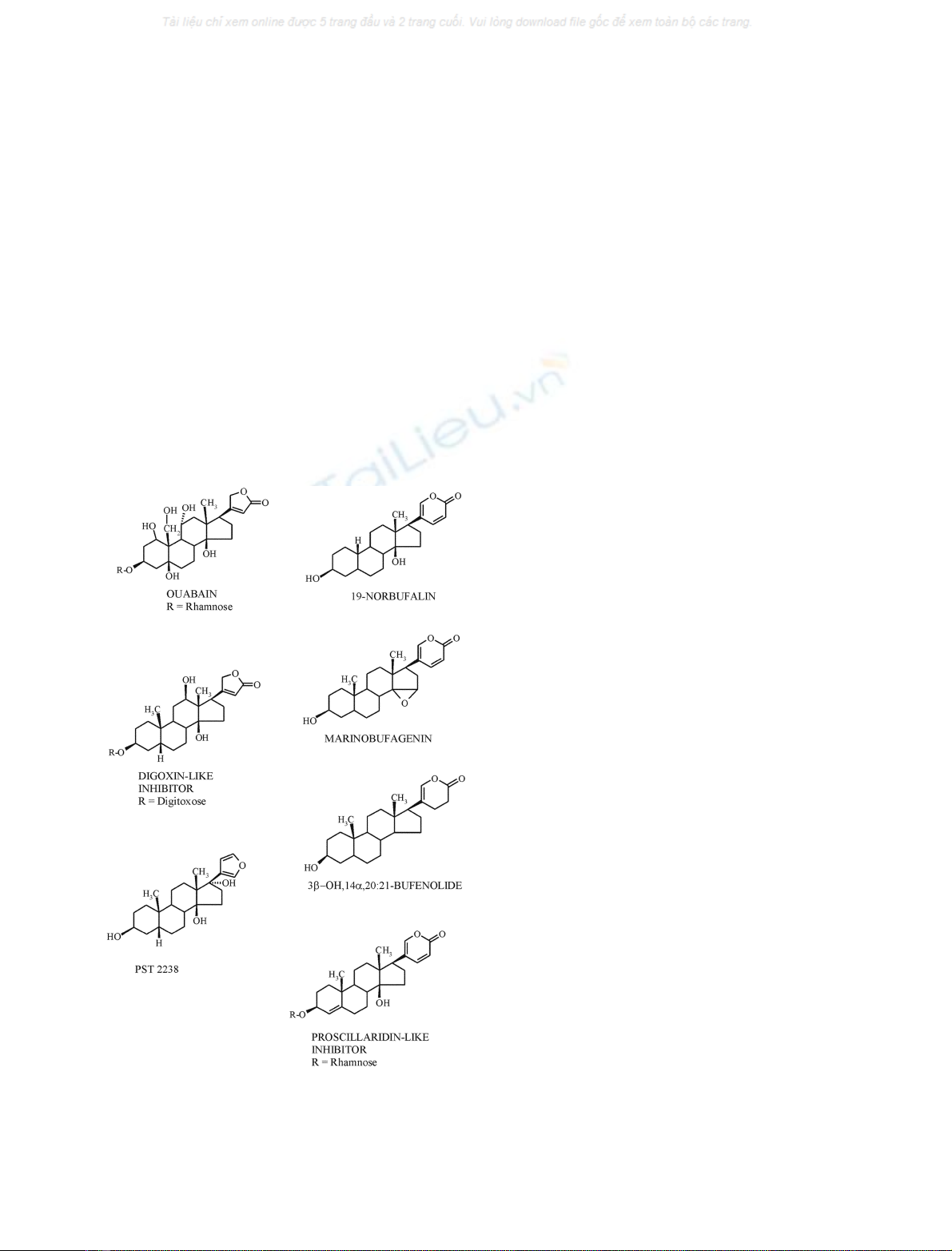
identification of endogenous ÔdigitalisÕas a group of
cardenolides and bufadienolides whose physiological and
pathophysiological function is only starting to be under-
stood. Readers who are interested in more detailed infor-
mation are referred to recent reviews [9,14–17].
OUABAIN IS A NEW STEROID
HORMONE
Identification of ouabain as endogenous inhibitor
of the sodium pump
There is now much evidence that ouabain is a new steroid
hormone of the adrenal cortex and hypothalamus (Fig. 1).
Ouabain-like immunoreactivity has been found in almost all
tissues, including plasma, but the highest concentrations
have been observed in the adrenal, hypophysis, and
hypothalamus [16,18,19]. The long-standing dispute as to
whether endogenous ouabain is identical to plant-derived
ouabain or whether it is an isomer thereof is turning in favor
of a structure identical to the plant-derived ouabain [20–22].
A substance with striking similarity to ouabain was
previously isolated from human urine [23] and bovine
adrenals [24]. Hamlyn and his coworkers isolated 10 lgof
ouabain or its isomer from 85 L of human plasma [18,25].
The compound of 585 295 Da in fast atom bombardment
mass spectrometry (FAB-MS) gave very similar results in a
direct comparison with ouabain in linked tandem MS, after
derivatization with acetic anhydride coupled with FAB-MS
and in analytical HPLC that was able to detect an altered
stereochemistry of a single sugar OH group [25]. Hence, it
was concluded that the endogenous inhibitor of the sodium
pump is ouabain or a closely related isomer. Schneider et al.
however, were the first to show that ouabain is in fact a
constituent of the adrenals [21]. They isolated 20 lgofa
pure substance from 20 kg of bovine adrenals and identified
the substance by ESI-MS and
1
H-NMR spectroscopy as
ouabain [21]. The hypothalamic inhibitor of the sodium
pump from bovine brain was considered for a long time to
be an isomer of ouabain, but it was determined recently that
the previous microanalysis was erroneous because of the
presence of borate, which had diffused out of the borosili-
cate glass used to store the minute amount of pure
substance. Hence, it was demonstrated beyond doubt that
the inhibitor from bovine hypothalamus is also ouabain
[20]. In summary, ouabain, an arrow poison of the African
Ouabaio tree and of Strophanthus gratus plants and a long-
known inhibitor of the sodium pump, has been identified in
blood plasma, adrenal glands, and the hypothalamus of
mammals.
The adrenal gland as a source of ouabain
The surprising observation that a plant toxin can be purified
from mammalian sources leads to the question as to
whether this substance was taken up from the diet and
consequently stored in the tissues where it has been
identified. In fact, orally and parenterally administered
ouabain is selectively taken up by the adrenal [26], although
intestinal uptake of ouabain is considered to be only 3–5%
of administered substance [27]. Ouabain is also taken up by
cultured adrenal cells [28]. Consistent with the adrenal gland
being a major place of synthesis and/or storage of ouabain,
adrenalectomy leads to a lowering of ouabain plasma levels
[18,29]. The adrenal cortex is likely to be the place of storage
and/or synthesis, because it contains more ouabain than the
medulla [19] and demedullectomized rats show no lowering
of their plasma concentrations of ouabain when compared
with sham-operated controls [16]. Adrenal glands of con-
scious dogs release ouabain [30]. It is not known whether
storage vesicles exist that release ouabain upon hormonal
stimulus.
Biosynthesis of cardenolides in mammals
Ouabain is apparently synthesized in the adrenals and
released upon hormonal stimulation. Extirpation of an
adrenocortical tumor overproducing ouabain, a ouabai-
noma, in a human patient reverted the elevated blood
pressure to normal [31]. In another case, compression of the
tumor during extirpation of a pheochromocytoma increased
the patient’s plasma level of ouabain immunoreactivity and
norepinephrine. The tumor had higher tissue concentrations
of ouabain than the normal adrenal cortex [32]. De novo
synthesis of ouabain and dihydro-ouabain has been
Fig. 1. Structures of endogenous cardiotonic steroids that have been
isolated in the search for ‘endogenous digitalis’. Compounds with an
unsaturated five-membered lactone ring are cardenolides and those
with an unsaturated six-membered lactone ring are bufadienolides.
PST 2238 is a ouabain antagonist [75,76].
ÓFEBS 2002 Endogenous cardiac glycosides (Eur. J. Biochem. 269) 2441

demonstrated in tissue culture experiments [33]. Bovine
adrenocortical cells in vitro secrete ouabain in amounts that
exceed their cell content by up to tenfold [28,34,35]. The
biosynthesis occurs in zona fasciculata cells. Bovine adreno-
cortical cells in tissue culture release ouabain upon exposure
to adrenocorticotropin, a1-adrenergic receptor agonists,
and angiotensin II [36–38]. Human CLR7050 cells (an
adrenal cortex-derived cell line) are insensitive to adreno-
corticotropin and angiotensin II but sensitive to arginine
vasopressin and phenylephrine [38]. The phenylephrine-
dependent release of ouabain from human CRL7050 and
bovine adrenocortical cells in culture is blocked by the
a1-adrenergic receptor antagonist doxazosin. This was
interpreted to indicate that the sympathetic nervous system
is involved in regulation of the release of this hormone to the
bloodstream [38]. In bovine adrenal cortical cells, angioten-
sin II acts via the angiotensin type 2 (AT
2
) receptor, because
the AT
2
agonist CGP42112 stimulates the release of
ouabain and the AT
2
antagonist PD123319 inhibits it [36].
However, a signaling pathway involving the brain’s O
2
chemoreceptor seems to exist as well, because hypoxia
triggers a marked release of the hypothalamic inhibitory
factor (ouabain) from midbrain and adrenals in rats [39,40].
More direct evidence for the existence of the biosynthetic
pathway in mammals comes from the demonstration that the
administration of certain precursors increases the rate of
synthesis of the cardiotonic steroid or, if given in their
radioactive form, leads to the formation of radioactive
cardiotonic steroids. Progesterone and pregnenolone have
been shown to be precursors of endogenous ouabain
[16,22,35]. In addition, rhamnose could readily enter adreno-
cortical cells and increase the biosynthesis of endogenous
ouabain [35]. Inhibition of the 3b-hydroxysteroid dehydro-
genase that forms progesterone stimulates the secretion of
endogenous ouabain [16] similar to that seen with the
addition of progesterone itself [35]. Hence, it seems possible
that various 3b-hydroxysteroid dehydrogenase isoforms are
involved in the biosynthesis of aldosterone and ouabain.
When [7-
3
H]pregnenolone is added to primary rat adrenal
cells, radioactivity is found in a fraction with digitalis-like
activity but not with ouabain [41]. At present, it is unclear
why different cell systems yield different results. There is no
doubt, however, that biosynthesis of the cardenolides
ouabain or digitalis occurs in adrenocortical cells.
PHYSIOLOGY AND PATHOPHYSIOLOGY
OF ENDOGENOUS OUABAIN
Regulatory short-term effects
We are only starting to obtain information on the physiology
and pathophysiology of endogenous ouabain. Recently, we
reported that submaximal treadmill exercise of dogs rapidly
increased the concentrations of ouabain in blood by about
50- to 500-fold. Upon rest, ouabain levels fell with a half-life
of 5–8 min. Pretreatment of dogs with the b-blocker atenolol
as well as the angiotensin converting enzyme (ACE) inhibitor
benazepril abolished the exercise-dependent rise in endo-
genous ouabain levels, indicating that the release of ouabain
in dogs is under the control of epinephrine and angiotensin II
[42]. adrenocorticotropin did not stimulate ouabain release
in man [43] or dogs [42]; but was effective in rats [44].
Similarly, ergometric training of normotensive human
volunteers led to a rapid increase in endogenous ouabain
concentrations that declined rapidly upon rest [45]. The
observations of a rapid rise and decline of endogenous
ouabain upon physical exercise are consistent with the
properties of a fast-acting, circulating hormone.
Regulatory long-term effects
Elevated concentrations of endogenous ouabain (ouabain-
like immunoreactivity) have been found under a number of
conditions such as sodium imbalance, chronic renal failure,
hyperaldosteronism, congestive heart failure, and pre-
eclampsia [14,16,46,47]. Ouabain has been shown to produce
vasoconstriction in man at low doses. The most striking
finding in humans is that approximately 50% of Caucasians
with uncomplicated essential hypertension show increased
concentrations of endogenous ouabain, reduced heart rate,
and greater left ventricular mass and stroke volume of the
heart [48]. Circulating levels of endogenous ouabain corre-
late directly with mean blood pressure, relative thickness of
the left ventricular heart wall, and the total peripheral
resistance index [49,50]. Immunization of rats against
ouabain lowers arterial blood pressure [16] as does infusion
of the commercially available Fab fragment of an anti-
digoxin Ig (Digibind), which cross-reacts with ouabain, in
humans and rats [51,52]. Exposure of rats for a long period
to small (nanomolar) doses of ouabain or other cardeno-
lides leads to hypertension [53–56]. The hypertensinogenic
action of ouabain was also observed with ouabagenin,
dihydro-ouabain, iso-ouabain (containing an oxygen bridge
between C14 of the steroid and C21 in the lactone ring), and
an lactone ring-opened ouabain. Unexpectedly, however,
the hypertensinogenic effect of these ouabain derivatives
increased with the decrease in the potency of the compounds
to inhibit Na
+
/K
+
-ATPase; i.e. an inverse linear relation-
ship between the rise in blood pressure and the logarithm of
the IC
50
was observed [57]. Also surprising, although
consistent with clinical experience, was that digoxin was
unable to raise blood pressure [48,58]; on the contrary, it
lowered it [57]. Moreover, digoxin and digitoxin reduce the
hypertensive effect of ouabain [48,59]. The observation that
the hypertensinogenic activity of cardiac glycosides is not
directly related to their potency as inhibitors of Na
+
/K
+
-
ATPase raises the possibility that the sodium pump may not
be the initial target in the mechanism by which ouabain
induces a sustained increase in blood pressure. The hyper-
tensinogenic activity of ouabain and its analogs may arise
from a novel mechanism linked with the steroid nucleus [57].
It has been known for some time that treatment of cells in
culture with cardiac glycosides affects cell proliferation as
well as the expression of isoforms of Na
+
/K
+
-ATPase.
When infused for 6 weeks in rats, ouabain and digoxin
differentially affected the expression of isoforms of the
sodium pump in different tissues [58]. The molecular
mechanism by which ouabain affects cell differentiation
has been studied in heart muscle in great detail by Xie &
Askari and their coworkers [60]. Therapeutic concentrations
of ouabain stimulate the growth of muscle cells and protein
biosynthesis, including the Ca
2+
-dependent expression of
the early response genes c-fos and c-jun as well as Ras and
p42/44 mitogen-activated protein kinases, which are viewed
as key mediators of cardiac hypertrophy [60]. In rat renal
epithelial cells, ouabain causes low-frequency intracellular
2442 W. Schoner (Eur. J. Biochem. 269)ÓFEBS 2002
calcium oscillations at concentrations that partially inhibit
the sodium pump. These oscillations are caused by a
concerted action of InsP
3
receptors and capacitive calcium
entry via plasma membrane channels. The low-frequency
intracellular oscillations elicit an activation of the transcrip-
tion factor, NF-jB [61]. It is well known that changes in
monovalent ion fluxes are also intimately connected to the
processes of mitogenesis and differentiation. Consistent
with a central role of the sodium pump in these processes,
ouabain selectively inhibits interferon a-induced gene
expression [62], induces mRNAs encoding the growth
factors interleukin 6 and granulocyte macrophage colony-
stimulating factor [63,64], and increases c-fos and c-jun
expression in a variety of cultured cells [62]. Because the
expression of c-fos is regulated by a number of hormones,
growth factors, and mitogens associated with cell prolifer-
ation and differentiation, it is not surprising that other
cardiotonic steroids, namely bufalin and bufalin-like fac-
tors, also affect cell differentiation [65–67].
Ouabain, a neurosteroid, mediates sympathetic
hyperactivity in salt-sensitive hypertension
Ouabain has been identified in the hypothalamus and it is
present in the pituitary and in medullary neurons. Pituitary
ouabain is considered to be released into the circulation but
paracrine secretion may occur. Cold-induced brain edema
in cats leads to a significant increase in ouabain-like activity
in the cerebrospinal fluid and the edematous brain hemi-
sphere [68]. In conscious rats, acute intracerebroventricular
injection of ouabain or crude hypothalamic or pituitary
extracts containing ouabain-like activity causes similar
increases in sympathetic activity, blood pressure, and heart
rate. These effects can be prevented by the simultaneous
intracerebroventricular administration of Fab fragments of
Digibind, which cross-react with ouabain [69,70]. The
effects of central Na
+
and ouabain are attenuated in
transgenic rats that are deficient in brain angiotensinogen
[71]. In normal rats, sympathetic hyperactivity and hyper-
tension induced by chronic ouabain and hypertonic saline
treatment is prevented by angiotensin type 1 receptor
blockade [72]. High salt intake also increases the expression
and activity of the ACE in hypothalamus and pons of Dahl
salt-sensitive rats without a parallel increase in angioten-
sin II levels. Chronic blockade of brain ÔouabainÕby
intraventricular infusion of a ouabain-binding antibody
lowered the NaCl-dependent rise in the amount of ACE
mRNA, which may indicate that the increase in ACE
mRNA is secondary to the activation by brain ÔouabainÕ
[73]. Presently, it is unclear how inhibition of the sodium
pump in brain cells affects ACE expression. It is conceivable
that inhibition of the pump leads to an increase in
intracellular Ca
2+
concentrations that in turn lead to an
increase in ACE expression and activity, as has been shown
for ACE expression induced by platelet activating factor
and endothelin [74]. A tentative regulatory scheme based on
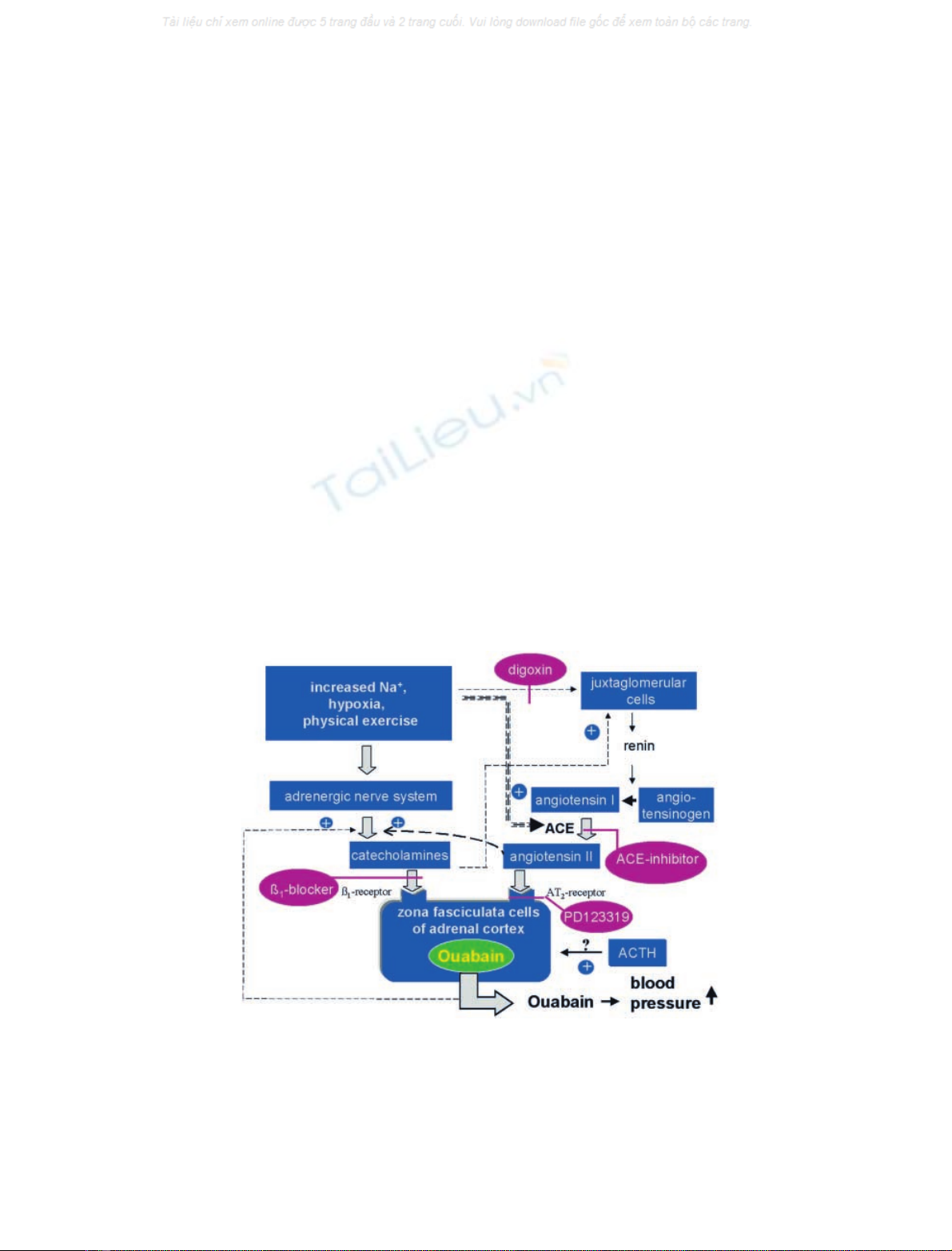
the information available thus far is shown in Fig. 2.
Anti-ouabain as an antihypertensinogenic agent
Ouabain-induced hypertension is believed to be mediated
via inhibition of the sodium pump. Therefore, ouabain
Fig. 2. Hormonal control of the release of endogenous ouabain from zona fasciculata cells of the adrenal cortex by hypernatriaemia, hypoxia, and
physical exercise. The scheme represents a compilation of the findings that ouabain levels are increased in patients with low-renin hypertension [50],
in hypoxemia [39,40], and during physical exercise [42]. In adrenocortical cells, ouabain is released by phenylephrine and angiotensin II, and its
action is blocked by the AT
2
inhibitor PD123319 [36–38]. The ACE inhibitor prazolol and the b-blocker atenolol abolish the exercise-dependent rise
in ouabain levels in dogs [42]. Digoxin counteracts the ouabain-dependent rise in blood pressure in rats [48,57,59] and lowers renin levels in humans
[86]. It has been shown that ouabain and angiotensin II stimulate the release and synthesis of catecholamines and that catecholamines stimulate the
release of renin. Increased concentrations of Na
+
increase the synthesis of angiotensin converting enzyme (ACE) [107]. Stimulation of ouabain
release by adrenocorticotropin has been observed in tissue culture [36] and in vivo in the rat [44], but not in dog and man [42,43].
ÓFEBS 2002 Endogenous cardiac glycosides (Eur. J. Biochem. 269) 2443

antagonists may lower blood pressure. In fact, an new
compound resembling cardiotonic steroids, PST 2238,
shows such an antihypertensive action [75,76] (Fig. 1).
Micromolar concentrations of PST 2238 lower the ouabain-
induced hypertension in rats when given orally. This new
prototype of an antihypertensive drug acts also in Milan
hypertensive rats, where a genetic alteration of adducin
genes is associated with hypertension and up-regulation of
renal Na
+
/K
+
-ATPase. Hence, PST 2238 might be used for
the treatment of human essential hypertension caused by an
alteration of the cytoskeletal protein adducin [77].
Identification of other cardenolides as endogenous
inhibitors of the sodium pump
A number of observations indicate that additional cardio-
tonic steroids of the cardenolide or bufadienolide group
may play a role in the circulation (Fig. 1) [19,21,78,79]. The
existence of an endogenous digoxin can not excluded so far.
Approximately 7.9 lg of a substance indistinguishable from
digoxin was isolated from 100 tons of human urine [80]. Its
properties in FAB-MS, proton NMR, several different
HPLC systems and in its reactivity with digoxin antibodies
were identical with digoxin [81], but a digoxin-like immuno-
reactive factor from bovine adrenals appears to be slightly
different. This latter factor exists in a deglycosylated and a
reduced form [82,83]. It was found in blood plasma, urine,
adrenal glands, and breast cyst fluid [17]. There is, however,
no evidence so far that digoxin is synthesized in mammalian
cells. It may be taken up from the gut with the diet and
stored in the adrenals [26]. Endogenous digoxin immuno-
reactivity is increased in renal failure, in newborn infants,
and under conditions of hypertensive pregnancy as well as
during prolonged, strenuous exercise [15,17,84]. Plasma
digoxin immunoreactivity is increased about 2.5-fold in
patients with acute myocardial infarction [85]. Most inter-
esting is that digoxin counteracts the hypertensinogenic
effect of ouabain in rats [48,59] (Fig. 1). This may be due to
a decrease in plasma renin activity and angiotensin II,
aldosterone, and epinephrine levels, and a significant
increase in the levels of atrial and brain natriuretic peptides
[86]. Additionally, the digoxin-induced arterial baroreflex
opposes the sympathetic excitatory pressor responses to
ouabain in the periphery and in the brain [48,59,87] and
digoxin no longer activates the chemoreflex in patients with
chronic heart failure [88] (Fig. 2). It is thus unclear how two
substances that are both specific inhibitors of the sodium
pump can produce opposing physiological effects. One
reason may lie in the higher hydrophobicity of digoxin as
compared with ouabain, which would lead to a different
tissue distribution. However, other reasons may also exist.
Identification of bufadienolides as endogenous
inhibitors of the sodium pump
In addition to the endogenous cardenolides ouabain and
digoxin, endogenous bufadienolides have also been identi-
fied (Fig. 1) [19,78,89–91]. Material cross-reacting with
antibodies against the bufadienolides bufalin and proscil-
laridin A has been found to increase in concentration in
blood and to correlate with systolic blood pressure [79,92].
Bufalin-like immunoreactivity rose in the serum of Dahl-S
rats under the conditions of a high-salt diet [92]. Proscillar-
idin A-immunoreactive material was purified from bovine
adrenals. The compound, with a molecular mass of 600 Da,
had a UV maximum at 250 nm, which indicates that this
hydrophilic compound differs from a classical bufadienolide
with a UV maximum at 300 nm [21]. Another compound,
3b-hydroxy 14a20:21-bufenolide, has been purified and
identified from human placenta [78].
Is marinobufagenin, an endogenous a
1
sodium pump
inhibitor, a natriuretic hormone?
Marinobufagenin (3b,5b-dihydroxy-14,14-epoxybufadieno-
lide) (Fig. 1) was originally discovered in amphibians and
more recently isolated from the urine of patients with
myocardial infarction [90]. In contrast to ouabain, marino-
bufagenin exhibits a greater affinity for the ouabain-
resistant a1 subunit of Na
+
/K
+
-ATPase [93,94]. As one
of the factors associated with blunted natriuresis, salt-
sensitive Dahl rats have a mutation in the a1 subunit of
Na
+
/K
+
-ATPase [95]. The mutated renal sodium pump
exhibits an abnormal Na
+
/K
+
-ATPase pumping ratio,
which upon a high salt intake results in an inability of the
kidneys to fully excrete sodium [96,97]. When salt-sensitive
Dahl rats were exposed to an acute NaCl load, a transient
increase in the plasma endogenous ouabain concentration
was observed that was accompanied by a sustained increase
in the level of endogenous marinobufagenin [94]. It was
suggested that the increased blood plasma marinobufagenin
concentration is due to secretion that promotes natriuresis
and compensates for the genetically impaired pressure
natriuretic mechanism [98]. The bufadienolide is vasocon-
strictive [90]; it is elevated in volume expansion and pre-
eclampsia and, like ouabain, is increased upon voluntary
hypoventilation of human volunteers [99].
19-Norbufalin, a cardiotonic steroid causing cataract
formation
In normal lenses, immunoreactivity against bufalin and
ouabain-like factor is sevenfold to 30-fold higher in the
capsular epithelial layer than in the lens fiber region [100].
In human cataractous lenses, the concentration of the
sodium pump inhibitor was much higher than in normal
lenses. Hence, it was isolated from cataractous lenses and
identified as 19-norbufalin (Fig. 1) and its Thr-Gly-Ala
tripeptide derivative [91]. Cardiac glycosides alter the
osmotic balance of lenses and induce cataract formation
by crystalline degradation and protein leakage that initiate
opacity. Inhibition of the sodium pump in rat lenses down-
regulates the expression of the intracellular signaling protein
14-3-3 without a significant change in c-crystalline gene
expression. Because 14-3-3 proteins are multifunctional
regulatory proteins, a reduction in the abundance of
various isoforms would have profound effects on cell
function [101].
BINDING OF CARDIOTONIC STEROIDS
TO SERUM PROTEINS
As hydrophobic substances, steroid hormones are trans-
ported in blood as complexes bound to specific binding
globulins [102]. Serum albumin is presumed to fulfill this
role for cardiac glycosides but at concentrations of the
2444 W. Schoner (Eur. J. Biochem. 269)ÓFEBS 2002


























