Production and chemiluminescent free radical reactions of glyoxal in
lipid peroxidation of linoleic acid by the ligninolytic enzyme,
manganese peroxidase
Takashi Watanabe
1
, Nobuaki Shirai
2
, Hitomi Okada
1
, Yoichi Honda
1
and Masaaki Kuwahara
1
1
Laboratory of Biomass Conversion, Wood Research Institute, Kyoto University, Gokasho, Uji, Japan;
2
Industrial Research Center of Shiga
Prefecture, Ritto, Kamitoyama, Japan
Glyoxal is a key compound involved in glyoxal oxidase
(GLOX)-dependent production of glyoxylate, oxalate and
H
2
O
2
by lignin-degrading basidiomycetes. In this paper, we
report that glyoxal was produced from a metabolite of
ligninolytic fungi, linoleic acid, by manganese peroxidase
(MnP)-dependent lipid peroxidation. In the absence of the
parent substrate of linoleic acid, the dialdehyde was
oxidized by MnP and Mn(III) chelate to start free radical
reactions with emission of chemiluminescence at 700 –
710 nm. The spectroscopic profile of the light emission is
distinguishable from (a) singlet oxygen, (b) triplet carbonyls
from dioxetane and a-hydroxyperoxyl radicals, and (c)
biacyl triplet formed by the coupling of two acyl radicals.
The photon emission of glyoxal by MnP was activated by
co-oxidation of tartrate. The MnP-dependent oxidation of
glyoxal in tartrate buffers continued for 10 days without
addition of exogenous H
2
O
2
. The importance of these
results is discussed in relation to the free radical chemistry
of lignin biodegradation by wood rot fungi.
Keywords: Manganese peroxidase; lipid peroxidation;
Ceriporiopsis subvermispora; acyl radical.
Lignin biodegradation by white rot fungi is an extracellular
chemical event generating free radicals. Lignin-degrading
enzymes, lignin peroxidase (LiP), manganese peroxidase
(MnP) and laccase (Lac), play a key role in generating free
radicals from lignin and oxidizable fungal metabolites such
as oxalate, glyoxylate, malonate, hydroquinones and aryl
alcohols. Due to the participation of peroxidases in the
lignin breakdown, a supply of hydrogen peroxide is essential
to drive the extracellular enzymatic process. So far, several
oxidases have been proposed as the enzymes which carry
out this task. The finding that glyoxal and glyoxal oxidase
(GLOX) are secreted by white rot fungi strongly suggests
that the GLOX system plays a key role in the extracellular
H
2
O
2
production [1–6]. As GLOX is activated by
peroxidases, the peroxidase-dependent lignin-degradation
can be controlled by the combination of GLOX and its
substrate, glyoxal [2,7]. Thus, the importance of glyoxal
oxidation in wood decay has been recognized. However,
little is known about the biosynthetic route for the extra-
cellular production of glyoxal by wood rot fungi. In this
paper, we first report that a ligninolytic enzyme, MnP, is able
to catalyze formation of glyoxal from a metabolite of wood
rot fungi, linoleic acid [8], by lipid peroxidation. The
glyoxal produced by MnP can be converted to glyoxylate
and oxalate by GLOX [6] and these carboxylic acids are
further oxidized by MnP or LiP/VA to yield O
2
†
–
and
CO
2
†
–
, which in turn reduce free radicals and transition
metals like Fe(III) [9–12]. Thus, the present result
highlights the new roles of MnP-dependent lipid peroxi-
dation in free radical chemistry of wood rot fungi.
In lipid peroxidation of USFAs, it has been reported that
Mn(II) reacts with a chain-carrying radical, peroxyl radical
(LOO†), to terminate the chain reactions [13,14]. This
raises the question of how the MnP-lipid system generates
free radicals in the presence of antioxidant, Mn(II).
Recently, we reported that the chain-braking antioxidative
activity of Mn(II) is suppressed by regeneration of free
radicals by breaking down of LOOH with MnP [15]. In this
process, we found that acyl radicals were predominantly
formed. This suggests that hydrogen abstraction from
aldehydes is involved in the major chain propagation
reactions of the MnP-dependent lipid peroxidation. The
observation of acyl radicals in the MnP/lipid system
prompted us to analyze whether MnP can directly oxidize
the aldehyde intermediate in order to carry chain-reactions
Correspondence to T. Watanabe, Laboratory of Biomass Conversion,
Wood Research Institute, Kyoto University, Gokasho, Uji, Kyoto
611-0011, Japan, Fax: 181 744 38 3600,
E-mail: twatanab@kuwri.kyoto-u.ac.jp
Enzymes: manganese peroxidase (EC 1.11.1.13); lipoxygenase
[linoleate:oxygenoxidoreductase (EC 1.11.13)]; glyoxal oxidase
(EC 1.2.3.-).
(Received 8 May 2001, revised 24 September 2001, accepted
27 September 2001)
Abbreviations:O
2
†
–
, superoxide anion; CO
2
†
–
, formate anion
radical; MnP, manganese peroxidase; LiP, lignin peroxidase;
HRP, horseradish peroxidase; 13(S)-HPODE,
13(S)-hydroperoxy-9Z,11E-octadecadienoic acid; SFA, saturated fatty
acid; USFA, unsaturated fatty acid; 2,6-DMP, 2,6-dimethoxyphenol;
ESR, electron spin resonance; MDA, malondialdehyde; MSTFA,
N-methyl-N-trimethylsilyltrifluroacetamide; DFB, decafluorobenzene;
GLOX, glyoxal oxidase, TBARS, thiobarbituric acid reactive
substances; PFBHA, O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine
hydrochloride; PFBO, pentafluorobenzyl oxime; CH
3
CN, acetonitrile;
MeOH, methyl alcohol; EtOH, ethyl alcohol; DM, n-dodecyl
b-maltoside; DHMA, dihydroxymaleic acid; EI/GC/MS, electron
ionization-gas chromatography-mass spectrometer; PAH, polycyclic
aromatic hydrocarbon.
Eur. J. Biochem. 268, 6114–6122 (2001) qFEBS 2001

without the aid of the other oxidizable substrates. We now
report the formation and chemiluminescent chain reactions
of glyoxal in MnP-dependent lipid peroxidation of linoleic
acid.
MATERIALS AND METHODS
General methods
Manganese (II) sulfate and 1,2,3-trimethoxybenzene,
decafluorobenzene, 1-dodecanal, 1-decanal, 2,4-nonadienal,
1-hexanal, 1-nonanal, 1-pentanal, 1-octanal, 1-butanal,
glyoxlic acid, glycol aldehyde, glyoxal were obtained
from Wako Pure Chemical Industries (Tokyo, Japan).
trans,trans-2,4-Decadienal, cis-4-decenal was obtained from
Aldrich Chemical Company (Milwaukee, USA). trans-2-
Hexenal, 1-undecanal, 1-heptanal, trans-2-nonenal, 1-tride-
canal, 2-butanone, 3-buten-2-one, 3-pentanone, 2-pentanone,
2-heptanone, 2-hexanone, 2-octanone was obtained from
Tokyo Kasei Kogyo (Tokyo, Japan). Linoleic acid was
purchased from Nacalai Tescque (Kyoto, Japan). The linoleic
acid was purified by passing through a Sep-Pak
TM
CN Light
cartridge (Waters, Milford, MA, USA). After dissolving in
n-hexane, the eluent from the cartridge was evaporated with a
gentle stream of N
2
gas. Milli-Q
TM
water was used
throughout the experiments. All of the chemicals used were
of analytical reagent grade. 13(S)-Hydroperoxy-9Z,11E-oc-
tadecadienoic acid [13(S)-HPODE] was prepared as
described previously [15]. Malondialdehyde (MDA) was
synthesized as described previously [16].
Enzyme preparation
Crude MnP from Ceriporiopsis subvermispora FP-90031
was collected from 7-day-old cultures grown on a wood
medium composed of beech wood (5 g), glucose (0.7 g) and
peptone (0.7 g) at 28 8C. The culture filtrate was dialyzed
against 20 mMsodium succinate buffer (pH 4.5). The
dialyzate was concentrated by ultrafiltration, precipitated
with (NH
4
)
2
SO
4
and then purified by gel filtration on
Superdex 75 PG (1.6 60 cm, Amersham Pharmacia
Biotech, Sweden) using 20 mMsodium succinate buffer
containing 0.1 MNaCl as an eluent. Fractions showing MnP
activities were collected, and desalted with Centriprep
YM-30 (cut off, 30,000, Millipore, USA). MnP was further
purified by preparative IEF as described previously [15]
[pI 3.40, Reinheitzahl (RZ, Aat l
max
/A280) value: 3.0,
1.0 U ¼8.75 10
211
mol]. Low molecular mass com-
pounds were removed by successive washings with Milli-
Q
TM
water with a Centricut N-10 ultrafiltration concentrator
(cut off, 10 000, Kurabo, Japan) before use. For the time
course experiments of aldehyde production, the enzyme
purified on Superdex 75 PG was desalted with distilled
water in Centricut N-10 and used without further
purification (15 U:mL
21
). Laccase activity in the partially
purified fraction was below 0.02 U:mL
21
. Glyoxal oxidase
activity was not found in all the enzyme preparations.
Enzyme assay
MnP activity was measured with 2,6-DMP. The reaction
mixture contained 0. 2 mM2,6-DMP, 0. 5 mMMnSO
4
,
0. 1 mMH
2
O
2
,25mMsodium tartrate buffer (pH 3. 0) and
the enzyme solution. Reactions were started by adding H
2
O
2
and were quantified by monitoring the initial rate of
increase in absorbance at 470 nm in the presence and
absence of manganese. One unit of enzyme activity is
defined as the amount of enzyme that oxidizes 1 mmol of
2,6-DMP in 1 min. Laccase activity was measured
with 2,6-DMP under the same conditions but without
H
2
O
2
. Lipoxygenase activity was measured by O
2
uptake in
a reaction system containing 1. 5 mMlinoleic acid, 1 mM
n-dodecyl b-maltoside (DM) and 20 mMTris/HCl buffer
(pH. 9. 0). One unit of lipoxygenase activity is defined as
the amount of enzyme that absorbs 1 mmol of O
2
in 1 min.
GLOX activity was measured by O
2
uptake in a
reaction system containing 3 mMglyoxal in 20 mMsodium
tartrate (pH 3. 0), acetate (pH 4. 5) or phosphate (pH 6. 0)
buffers.
Electron ionization/gas chromatography/mass
spectrometetry (EI/GC/MS) analysis of oxidation
products by MnP
Linoleic acid and aldehydes were reacted with 250 mU of
the purified MnP, 0.5 mMof Mn(II) and 50 mMof H
2
O
2
at
20 8C for 1– 24 h in 10 mMacetate, formate, lactate and
tartrate buffers (pH 4.5). After the reaction, 0.5 mL of
aqueous PFBHA (0.05 M, 200 mL) was added and reacted at
35 8C for 0.5 h [17]. To this solution, 10 mL of a 10-mM
methanol solution of decafluorobenzene (DFB) and a
drop of 18-N-sulfuric acid were added and the mixture
was partitioned between n-hexane and H
2
O twice. The
hexane layer was dried over Na
2
SO
4
, evaporated with a
gentle stream of N
2
gas and directly injected into an EI/GC/
MS system. The EI/GC/MS analysis was done with a
Shimadzu QP-5050 A GC/MS with ionization energy of
70 eV on CP-Sil-8 (50 m 0.25 mm internal diemeter,
Chrompack, Netherlands) using helium as a carrier gas. The
column oven temperature was raised from 80 8C to 250 8C
at 5 8C:min
21
, and maintained at 250 8C for 20 min. The
time course of glyoxal production by MnP was analyzed as
described above after the reaction with and without linoleic
acid in formate and tartrate buffers. EI/GC/MS analyses of
authentic aldehydes and ketones were carried out using a
0.6-mMmethanol solution after derivatization with PFBHA
under the conditions described above. Tetramethylsilation
by N-methyl-N-trimethylsilyltrifluroacetamide (MSTFA)
was carried out as described previously [18].
Chemiluminescence measurements
Chemiluminescence was measured by an ultra-high sensi-
tive photon counter (ARGUS-50/VIM, Hamamatsu Photo-
nics, Hamamatsu, Japan) equipped with a charge-coupled
device (CCD) camera connected with an image intensifier
and ARGUS-50 image processor. The wavelength range of
the detector was 350–650 nm, 512 483 pixels, and the
noise count was 0.15 c.p.s. The reactions were carried out in
a cuvette for a 96-well microplate reader. The conditions for
each experiment are described in the figure legends.
Inactivation of MnP was carried out by heating the MnP
in a boiling water bath for 10 min
The chemiluminescence spectra were measured by a
simultaneous multiwavelength analyzer CLA-SP2 (Tohoku
Electronic Industries Co. Ltd, Sendai, Japan) with an
qFEBS 2001 Production of glyoxal in lipid peroxidation by MnP (Eur. J. Biochem. 268) 6115
incident slit width of 1.0 mm. The wavelength range of the
spectrometer was 370– 820 nm. Experimental conditions
are described in the legend of each figure.
RESULTS
Formation of glyoxal in the reaction of linoleic acid
with MnP
Lipid peroxidation by MnP is a free radical process capable
of decomposing recalcitrant PAH and nonphenolic lignin
model compounds [19–21]. We previously reported that the
oxidation of linoleic acid by MnP produced acyl radicals in
both tartrate and acetate buffers [15]. The formation of acyl
radicals strongly suggests that hydrogen abstraction from
aldehyde [22,23] is involved in the oxidative process. To
analyze the aldehydes formed by this reaction, linoleic acid
was reacted with MnP for 19 h at 20 8C in sodium acetate,
formate, lactate and tartrate buffers and the oxidation
products were analyzed by EI/GC/MS after derivatization
to pentafluorobenzyloxims (PFBO) with PFBHA [17]. EI/
GC/MS analysis of the reaction products and authentic 19
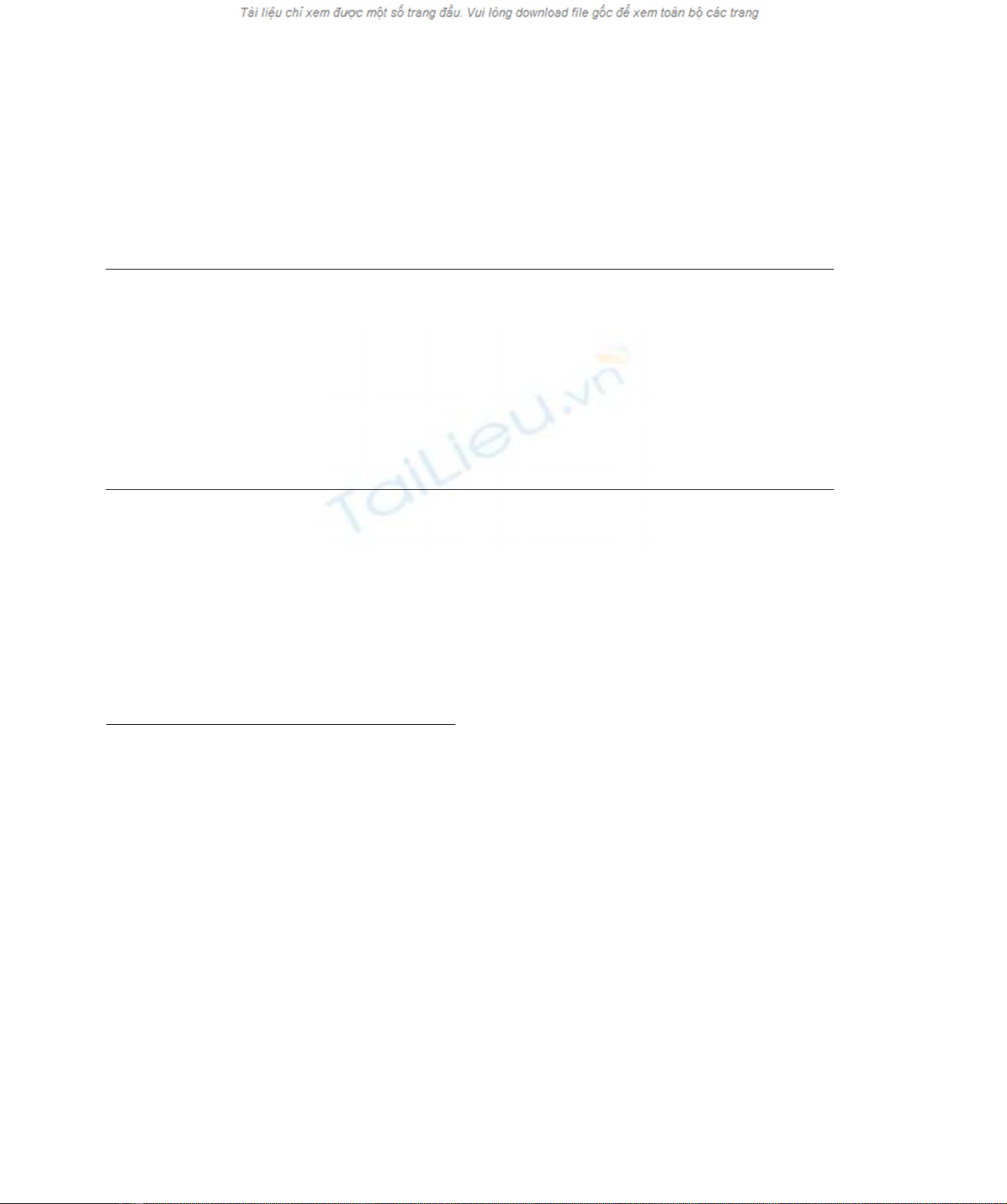
aldehydes and seven ketones demonstrated that glyoxal,
1-hexanal and 1-pentanal were formed from linoleic acid
by MnP in any of the buffer systems (Fig. 1). Syn and
anti-isomers of these PFBO derivatives were separated on
the GC/MS column. The mass spectrum of PFBO
derivatives of glyoxal formed from linoleic acid is shown
in Fig. 2, together with that of authentic standard. MDA, a
major peroxidation product derived from polyunsaturated
fatty acids was not detected in the reaction products of MnP
in contrast to the oxidation of linoleic acid by xantine/
xanthinoxidase/Fe(II) [24]. The mass fragments of PFBO
derivatives characteristic to saturated aldehyde (m/z239),
2-enal (m/z250), 2,4-dienal (m/z276) and saturated
2-ketones (m/z72) [25], were not observed in the spectra
of unidentified carbonyl compounds, indicating that the
MnP/Mn(II)/lipid system proceeds by complex radical
reactions involving the formation of unusual carbonyl
species. Tetramethylsilation with MSTFA did not change
the mass chromatogram at m/z181 that originates from C –O
bond cleavage products of pentafluorobenzyl oxime [26]
(data not shown).
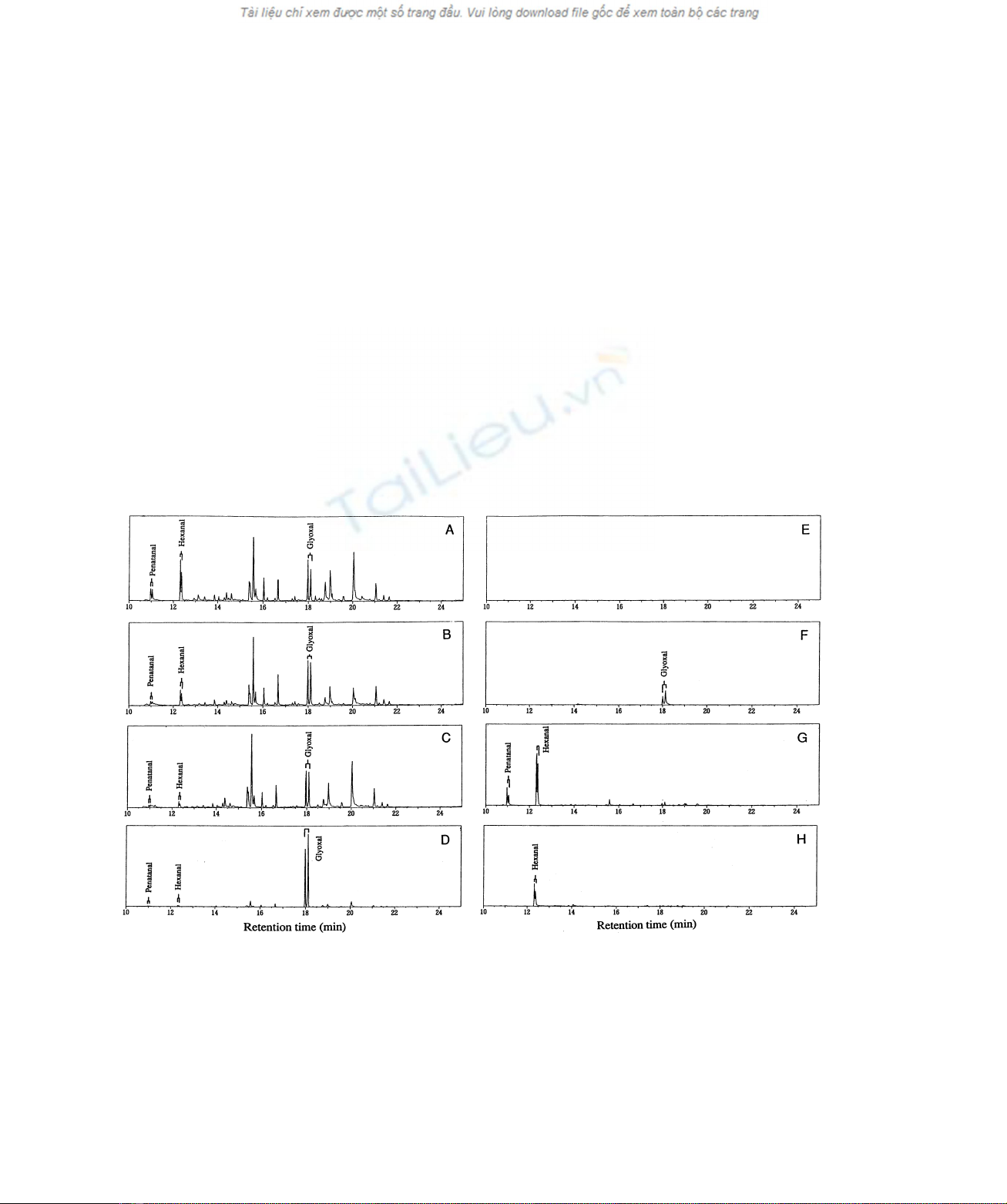
The reactions of MnP in four different buffers clearly
demonstrate that the formation of glyoxal was significantly
stimulated by the presence of tartrate. Therefore, the
reaction was carried out with and without linoleic acid in
sodium formate and tartrate buffers (Figs 1 and 3). GC/MS
analysis demonstrated that glyoxal was explosively
produced after 6 h in tartrate buffer containing linoleic
Fig. 1. Mass chromatograms of PFBO derivatives of products of lipid peroxidation by C. subvermipora MnP and soybean lipoxygenase at
m/z181. (A) Products of the oxidation of linoleic acid by MnP in sodium acetate buffer for 19 h. The reaction system (500 mL) contained 3 mM
linoleic acid, 500 mMMnSO
4
,50mMH
2
O
2
, 0.02% of Tween 20, 250 mU of purified MnP and 10 mMsodium acetate buffer (pH 4.5). (B) As (A) but
10 mMsodium formate buffer (pH 4.5) was used instead of acetate buffer. (C), As (A) but 10 mMsodium lactate buffer (pH 4.5) was used instead of
acetate buffer. (D) As (A) but 10 mMsodium tartrate buffer (pH 4.5) was used instead of acetate buffer. (E) As (B) but the reaction was carried out
without addition of linoleic acid. (F) As (D) but the reaction was carried out without addition of linoleic acid. (G) Products of the oxidation of linoleic
acid with soybean lipoxygenase. Linoleic acid (3 mM) was reacted with soybean lipoxygenase (10 U) in 40 mMTris/HCl buffer (pH 9.0) containing
0.02% of Tween 20 for 24 h at 20 8C. (H) Products of the oxidation of 13(S)HPODE by MnP in sodium lactate buffer (pH 4.5). for 3 h. The reaction
system contained 3 mM13(S)HPODE, 500 mMMnSO
4
,50mMH
2
O
2
, 0.02% of Tween 20, MnP (250 mU) and 10 mMsodium lactate buffer
(pH 4.5).
6116 T. Watanabe et al. (Eur. J. Biochem. 268)qFEBS 2001
acid. However, direct formation of glyoxal from tartrate was
also observed. In formate buffer, the production of glyoxal
was dependent on the presence of linoleic acid. The same
results were obtained with lactate and acetate buffers (data
not shown). In contrast to the oxidation of linoleic acid,
oxidation of 13(S)HPODE with MnP selectively produced
1-hexanal for 1–3 h (Fig. 1H). No PFBO-derivatives were
detected after the prolonged reaction of 13(S)HPODE. Thus
it was found that the formation of glyoxal was not catalyzed
by the direct oxidation of 13(S)HPODE with MnP.
Oxidation of linoleic acid with soybean lipoxyegnase
produced 1-hexanal and 1-pentanal (Fig. 1G).
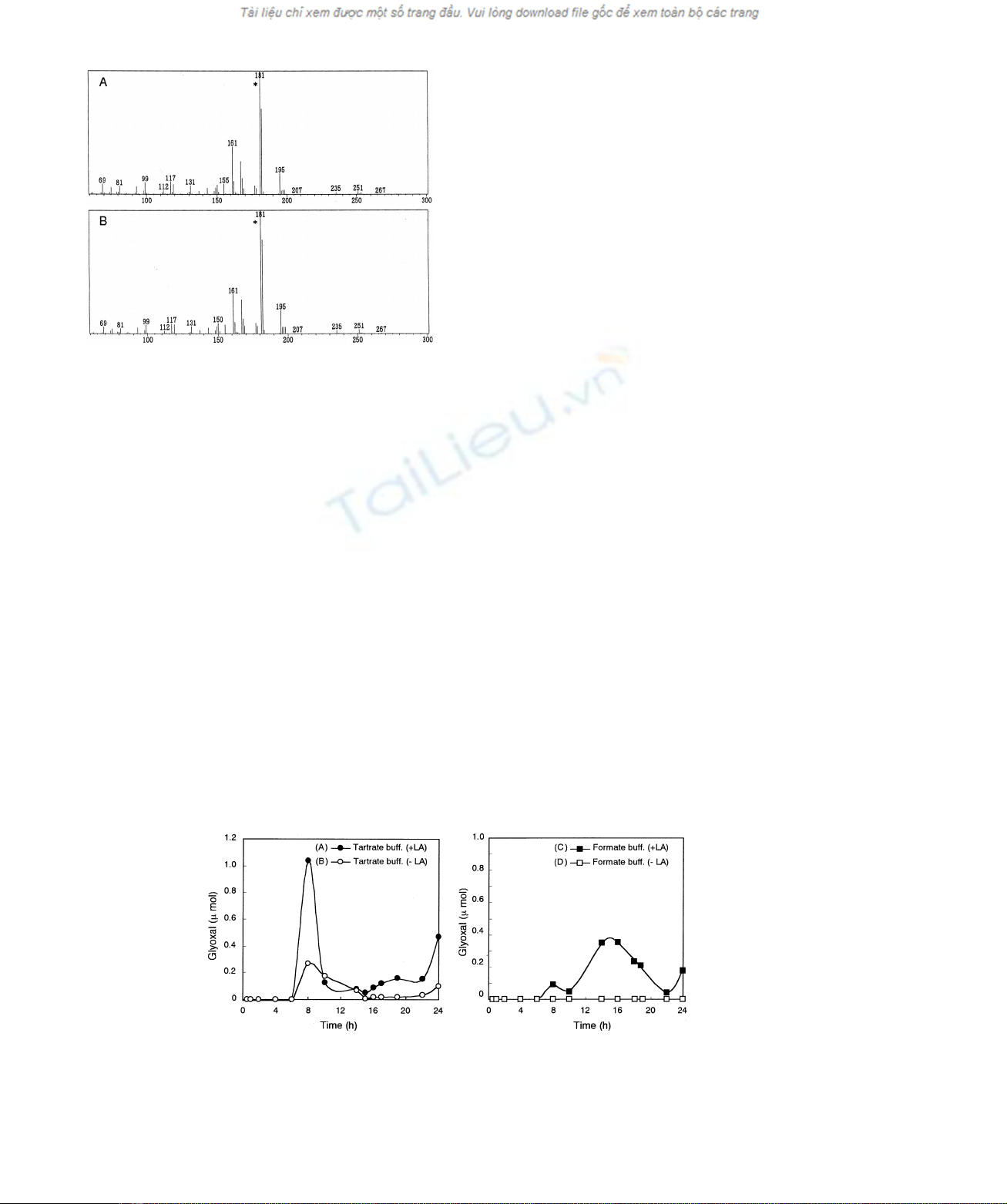
Emission of chemiluminescence in lipid peroxidation
The chemiluminescence detector is a powerful tool for
analyzing the oxidation of aldehydes due to its high
sensitivity and emission spectra characteristic to chemically
excited species. Therefore, oxidation of aldehydes and
linoleic acid by MnP was analyzed by a chemiluminescence
detector, in comparison with light emission by lipoxygenase
and the Fenton reaction (Fig. 4). Lipoxygenase is an enzyme
that abstracts hydrogen from the bis-allylic position of
unsaturated fatty acids containing cis,cis-1,4-pentadienyl
moiety. In the reaction with linoleic acid, the fatty acid is
oxidized to yield a pentyl radical [27] and 12-oxododecyl-
cis-9-enoic acid [28] via b-scission of hydroperoxide
intermediates, leading to production of 1-hexanal [29] and
1-pentanal as shown in Fig. 1G. When linoleic acid was
oxidized by soybean lipoxygenase, emission of chemilumi-
nescence was close to the background level, both in the
presence and absence of Fe(II). In the Fenton system,
chemiluminescence was also below the background level,
except for a weak emission of light from linoleic acid after 2
days (Fig. 4).
In contrast to these oxidation systems, reactions of glyoxal
with MnP in tartrate buffer emitted strong chemilumines-
cence. As shown in Figs 5 and 6, intensive light emission
was observed immediately after the reaction started. The
photon emission reached a maximum (35 000 counts:h
21
)
within 30 min, and then decreased, but chemiluminescence
of <9000 counts:h
21
was observed even after 1 h. In
lactate, formate and acetate buffers, the photon emission
was also observed within 30 min but the intensity was much
lower than that of the tartrate system. In the tartrate system,
the photon emission continued for 10 days, both in the
presence and absence of exogenous H
2
O
2
added initially
(Fig. 6). The photon emission from glyoxal was dependent
on the presence of Mn(II) and active enzyme. However, it
was found that the emission of chemiluminescence
continued for around 10 days when the reaction was started
without addition of glyoxal. This can be explained by the in
situ formation of glyoxal from tartrate with MnP (Fig. 1,3).
In the MnP-catalyzed oxidation of linoleic acid and the other
aldehydes, the light emission was not observed when Mn(II)
was omitted from the reaction system (data not shown).
When linoleic acid and seven different aldehydes were
reacted with Mn(III)–tartrate complex, strong light emission
was observed in the reaction system with glyoxal (Fig. 7).
The maximum photon emission intensity from glyoxal
reached 12 000 counts:h
21
. The photon emission was also
Fig. 2. Mass spectra of PFBO derivatives of glyoxal formed by the
oxidation of linoleic acid with MnP (A) and authentic standard (B).
(A) Glyoxal formed by the oxidation of linoleic acid with MnP for 19 h.
The reaction system (500 mL) contained 3 mMlinoleic acid, 500 mM
MnSO
4
,50mMH
2
O
2
, 0.02% of Tween 20, MnP (250 mU) and 10 mM
sodium acetate buffer (pH 4.5). (B) Authentic standard of glyoxal.
* 1/10 of the original signal intensity.
Fig. 3. Time course of glyoxal formation by MnP. (A) Glyoxal formed by the reaction of linoleic acid with MnP in sodium tartrate buffer. The
reaction system (500 mL) contained 3 mMlinoleic acid, 500 mMMnSO
4
,50mMH
2
O
2
, 0.02% of Tween 20, MnP (250 mU) and 10 mMsodium
tartrate buffer (pH 4.5). (B) As (A) but linoleic acid was omitted. (C) As (A) but 10 mMsodium formate buffer (pH 4.5) was used instead of sodium
tartrate bufer. (D) As (C) but linoleic acid was omitted.
qFEBS 2001 Production of glyoxal in lipid peroxidation by MnP (Eur. J. Biochem. 268) 6117
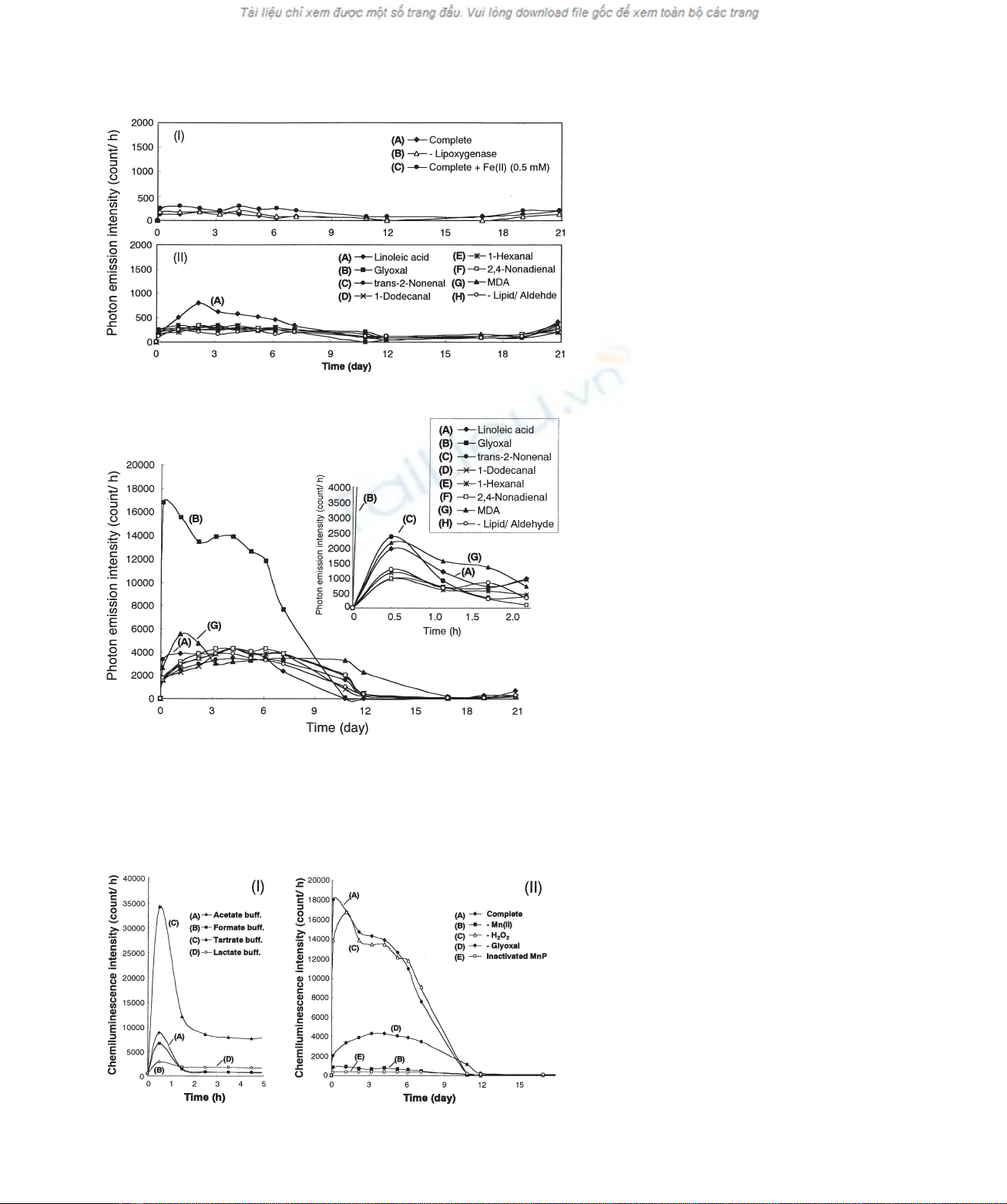
Fig. 4. Time course of light emission during
oxidation of linoleic acid by soybean
lipoxygenase (I) and the Fenton reaction (II).
(I): (A) The reaction system (200 mL) contained
4m
Mlinoleic acid, 10 U of lipoxygenase, 0.05%
of Tween 20, 10 mMTris/HCl buffer (pH 9.0). (B)
As (A) but lipoxygenase was omitted. (C) As (A)
but 0.5 mMFeSO
4
was added. II: (A) The reaction
system (200 mL) contained 4 mMlinoleic acid,
0.1 mMFeSO
4
, 0.2 mMH
2
O
2
, 0.05% of Tween 20.
(B) As (A) but glyoxal was added instead of
linoleic acid. (C) As (A) but trans-2-nonenal was
added instead of linoleic acid. (D) As (A) but
1-dodecanal was added instead of linoleic acid. (E)
As (A) but 1-hexanal was added instead of linoleic
acid. (F) As (A) but 2,4-nonadienal was added
instead of linoleic acid. (G) As (A) but MDA was
added instead of linoleic acid. (H) As (A) but
linoleic acid was omitted.
Fig. 5. Chemiluminescence emitted by the
oxidation of aldehydes and linoleic acid with
MnP in sodium tartrate buffer. (A) The reaction
system (200 mL) contained 4 mMlinoleic acid,
250 mU of MnP, 500 mMMnSO
4
, 0.2 mMH
2
O
2
,
0.05% of Tween 20 and 10 mMsodium tartrate
buffer (pH 4.5). (B) As (A) but glyoxal was added
instead of linoleic acid. (C) As (A) but
trans-2-nonenal was added instead of linoleic acid.
(D) As (A) but 1-dodecanal was added instead of
linoleic acid. (E) As (A) but 1-hexanal was added
instead of linoleic acid. (F) As (A) but
2,4-nonadienal was added instead of linoleic acid.
(G) As (A) but MDA was added instead of linoleic
acid. (H) As (A) but linoleic acid was omitted.
Inset shows the time course of the reactions (A–H)
during 2.5 h.
Fig. 6. Chemiluminescence emitted by the
oxidation of glyoxal with MnP. (I): (A) The
reaction system (200 mL) contained 4 mMglyoxal,
250 mU of MnP, 500 mMMnSO
4
, 0.2 mMH
2
O
2
,
0.05% of Tween 20 and 10 mMsodium acetate
buffer (pH 4.5); (B) As in (A) but 10 mMsodium
formate buffer (pH 4.5) was used instead of
sodium acetate bufer. (C) As (A) but 10 mM
sodium tartrate buffer (pH 4.5) was used instead
of sodium acetate bufer. (D) As (A) but 10 mM
sodium lactate buffer (pH 4.5) was used instead
of sodium acetate buffer.
(II): (A) The reaction system (200 mL) contained
4m
Mglyoxal, 250 mU of MnP, 500 mMMnSO
4
,
0.2 mMH
2
O
2
, 0.05% of Tween 20 and 10 mM
sodium tartrate buffer (pH 4.5). (B) As (A) but
MnSO
4
was omitted. (C) As (A) but H
2
O
2
was
omitted. (D) As (A) but glyoxal was omitted. (E)
As (A) but inactivated MnP was used instead of
native MnP.
6118 T. Watanabe et al. (Eur. J. Biochem. 268)qFEBS 2001



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





