Chemical structure and immunoreactivity of the lipopolysaccharide
of the deep rough mutant I-69 Rd
–
/b
+
of
Haemophilus influenzae
Sven Mu¨ ller-Loennies, Lore Brade and Helmut Brade
Research Center Borstel, Center for Medicine and Biosciences, Borstel, Germany
From the lipopolysaccharide of the deep rough mutant I-69
Rd
–
/b
+
of Haemophilus influenzae two oligosaccharides
were obtained after de-O-acylation and separation by
high-performance anion exchange chromatography.
Their chemical structures were determined by one- and
two-dimensional
1
H-,
13
C- and
31
P-NMR spectroscopy
as aKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1Pand
aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P. The spe-
cificity of mAbs S42-21 and S42-16 specific for Kdo-4Por
Kdo-5P, respectively [Rozalski, A., Brade L., Kosma P.,
Moxon R., Kusumoto S., & Brade H. (1997). Mol. Micro-
biol.23, 569–577] was confirmed with neoglycoconjugates
obtained by conjugation of the isolated oligosaccharides to
BSA. In addition, a mAb S42-10-8 with unknown epitope
specificity could be assigned using the neoglycoconjugates
described herein. This mAb binds to an epitope composed of
the bisphosphorylated glucosamine backbone of lipid A and
Kdo-4P, whereby the latter determines the specificity strictly
by the position of the phosphate group.
Keywords: carbohydrate antibody; Kdo-phosphate;
neoglycoconjugate; serology; sugar phosphate.
Haemophilus influenzae normally colonizes the human
nasopharynx but may cause severe infections, in particular
meningitis, in children. A major virulence factor of this
human pathogen is the type b capsule, an acidic polysac-
charide composed of ribose, ribitol and phosphate and
which is the basis of an effective conjugate vaccine [1].
Among other virulence factors is the lipopolysaccharide
(LPS) in which we are interested for various reasons: (a)
LPS is an essential component of the outer membrane in all
Gram-negative bacteria; (b) LPS is the endotoxin of Gram-
negative bacteria; (c) LPS is a major surface antigen leading
to the induction of protective antibodies; and (d) the
understanding of the biosynthesis of LPS may allow
the distinct blockage of essential steps as a new strategy
for the development of antibiotics [2,3].
The smallest LPS structure which still allows the bacter-
ium to survive was found in the mutant strain I-69 Rd
–
/b
+
of H. influenzae (referredtohereasI-69)wherea
single phosphorylated 3-deoxy-
D
-manno-oct-2-ulopyrano-
sonic acid (Kdo) residue is linked to the lipid A moiety.
Helander et al. have shown that the I-69 LPS was composed
of two molecular species with Kdo phosphorylated at either
position 4 or 5 [4].
The Kdo transferase of I-69 has been cloned and
characterized and the phosphokinase adding the phospho-
ryl group to position 4 of the Kdo residue has also been
cloned [5,6]. Coexpression of both enzymes in an Escheri-
chia coli strain lacking its own Kdo transferase led to the
synthesis of an LPS which contained exclusively Kdo-4P[7].
For this study mAbs were useful to identify the secondary
gene products. We have reported earlier on mAb recogni-
zing either the 4- or 5-phosphorylated Kdo which was
chemically synthesized and conjugated to BSA [8]. In
addition, we found mAb S42-10-8 which was specific for the
I-69 LPS but did not react with Kdo-4Por Kdo-5Palone.
Therefore, this antibody was assumed to recognize an
epitope requiring, in addition to a phosphorylated Kdo
residue, the phosphorylated lipid A backbone. As the LPS
species containing the Kdo-4Por Kdo-5Pcould not be
separated at that time and were not yet chemically
synthesized, the specificity of this mAb has not yet been
elucidated. Here, we report on: (a) the successful separation
of the deacylated carbohydrate backbone of I-69 LPS into
two pure oligosaccharides containing either Kdo-4Por
Kdo-5P; (b) the structural analysis of both oligosaccharides
by NMR; and (c) the characterization of a new mAb
recognizing a phosphorylated carbohydrate epitope.
MATERIALS AND METHODS
Bacteria and bacterial LPS
H. influenzae I-69 Rd
–
/b
+
was cultivated as described
previously [9]. Bacteria were washed with ethanol, acetone
(twice), and ether, and dried. LPS was extracted from dry
bacteria by the phenol/chloroform/petroleum ether method
[10] in a yield of 4.4% of dry bacteria. De-O-acylated LPS
was prepared after hydrazine treatment of LPS for 30 min
at 37 °C (yield: 81% based on the glucosamine content),
and deacylated LPS (LPS
deac
) was obtained by hydrolysis of
de-O-acylated LPS in 4
M
KOHasreported[11].LPS
deac
was further purified by preparative high performance anion
exchange chromatography (HPAEC) using water as eluent A
Correspondence to H. Brade, Research Center Borstel, Center for
Medicine and Biosciences, Parkallee 22, D-23845 Borstel, Germany.
Fax: + 49 4537 188419, Tel.: + 49 4537 188474,
E-mail: hbrade@fz-borstel.de
Abbreviations: HPAEC, high performance anion exchange chroma-
tography;Kdo,3-deoxy-
D
-manno-oct-2-ulopyranosonic acid; LPS,
lipopolysaccharide; LPS
deac
, deacylated LPS.
Note:S.Mu
¨ller-Loennies and L. Brade contibuted equally to this
work.
(Received 8 August 2001, revised 21 December 2001, accepted
3 January 2002)
Eur. J. Biochem. 269, 1237–1242 (2002) ÓFEBS 2002

and 1
M
ammonium acetate as eluent B and a gradient of
1% to 99% over 80 min. Desalting was achieved by gel
filtration on a column of 100 ·1.5 cm Sephadex G10 in
pyridine/acetic acid/water (4 : 10 : 1000, v/v/v) at a flow rate
of 1 mLÆmin
)1
. Fractions 1 and 2 were obtained in pure
form in yields of 21.6 and 9.5%, respectively, based on the
glucosamine content.
NMR spectroscopy
The deacylated LPS from H. influenzae I-69 was investi-
gated by one-dimensional
1
H-NMR- and
13
C-NMR and
spectroscopy at 600 and 150 MHz, respectively, on a Bruker
DRX 600 Avance spectrometer;
31
P-NMR spectra were
recorded on a Bruker DPX 360 Avance spectrometer at
145 MHz. All spectra were recorded on a 0.5-mL solution
of 5 mg sample in D
2
O. As reference served acetone
2.225 p.p.m. (
1
H), dioxane 67.4 p.p.m. (
13
C) and 85%
phosphoric acid 0 p.p.m. (
31
P). All spectra were run at a
temperature of 300 K. For
31
P measurements the pD was
adjusted to pD 2. Other measurements were performed at
pD 6 due to the acid labile nature of the Kdo-linkage.
Two-dimensional homonuclear
1
H,
1
H-DQF-COSY was
recorded over a spectral width of 7.5 p.p.m. in both
dimensions recording 512 experiments of 32 scans. Four
thousand data points were recorded in F2. Zero-filling
was applied in F1 to 1000 data points. Heteronuclear
1
H,
13
C-NMR correlation spectroscopy was recorded as
HMQC. Two thousand data points were recorded in F2
over a spectral width of 10 p.p.m. and 256 experiments
consisting of 24 scans per increment. Phase cycling was
performed using States-TPPI. Prior to Fourier transfor-
mation zero-filling was applied in F1 to 512 data points.
31
P-NMR spectroscopy was recorded with continuous
wave decoupling during acquisition. A total of 32 scans
was recorded. For
1
H,
31
P-NMR COSY a HMQC
experiment was recorded consisting of 256 experiments
and 32 scans each. Two thousand data points were
collected over a spectral width of 10 p.p.m. in F2 and
zero filling was applied in F1 to yield 512 data points. The
spectral width was 10 p.p.m. in F1.
Neoglycoconjugates
The amino groups of the glucosamine residues in LPS
deac
and in the oligosaccharides obtained from LPS
deac
were
activated with glutardialdehyde and conjugated to BSA as
described [12]. The amount of ligand present in the
conjugates was determined by measuring the amount of
protein (Bradford assay, Bio-Rad) and glucosamine
(Table 1).
MAbs
Monoclonal antibodies S42-16, S42-21 and S42-10-8 were
obtained after immunization and selection as described [8].
Culture supernatants were prepared in at least 100 mL
quantities and antibodies were purified on protein
G-Sepharose (Pharmacia/LKB) according to the supplier’s
instructions. Purification was ascertained by SDS/PAGE
and protein concentrations were determined by the bicin-
choninic acid assay (Pierce).
Serology
For ELISA, neoglycoconjugates were coated onto Maxi-
Sorp microtiter plates (U-bottom, Nunc). Antigen solutions
were adjusted to equimolar concentrations based on the
amount of ligand present in the respective glycoconjugate.
Unless stated otherwise, 50 lL volumes were used. Micro-
titer plates were coated with the respective antigen solution
in 50 m
M
carbonate buffer pH 9.2 at 4 °C overnight. Plates
were washed twice with distilled water; further washing was
carried out in NaCl/P
i
supplemented with 0.05% Tween 20
(Bio-Rad) and 0.01% thimerosal (NaCl/P
i
/Tween-T). Plates
were then blocked with NaCl/P
i
/Tween-T supplemented
with 2.5% casein (NaCl/P
i
/Tween-TC) for 1 h at 37 °Cona
rocking platform followed by two washes. Appropriate
antibody dilutions in NaCl/P
i
/Tween-TC supplemented
with 5% BSA were added and incubated for 1 h at 37 °C.
After washing, peroxidase-conjugated goat anti-(mouse
IgG) Ig (heavy and light chain specific; Dianova) was
added (diluted 1 : 1000) and incubation was continued for
1 h at 37 °C. After three washes in NaCl/P
i
/Tween-T, the
plates were washed in substrate buffer (0.1
M
sodium citrate,
pH 4.5). Substrate solution was freshly prepared and was
composed of azino-di-3-ethylbenzthiazolinsulfonic acid
(1 mg) dissolved in substrate buffer (1 mL) with sonication
in an ultrasound water bath for 3 min followed by the
addition of hydrogen peroxide (25 lLofa0.1%solution).
After 30 min at 37 °C, the reaction was stopped by the
addition of 2% aqueous oxalic acid and the plates were read
with a microplate reader (Dynatech MR 700) at 405 nm.
For ELISA using LPS as a solid-phase antigen another
protocol was used. Polyvinyl microtiter plates (Falcon 3911)
were coated with various amounts of LPS dissolved in NaCl/
P
i
(10 m
M
pH 7.3, 0.9% NaCl, 50 lL) at 4 °C overnight or
at 37 °C for 1 h. All following steps were performed at 37 °C
with gentle agitation and all washing steps were performed
four times. Coated plates were washed in NaCl/P
i
, blocked
for 1 h with blocking buffer (2.5% casein in NaCl/P
i
)and
then incubated for 1 h with mAb diluted in blocking buffer
(50 lL). Plates were washed in NaCl/P
i
and incubated for
Table 1. Oligosaccharides and neoglycoconjugates used in this study. For derivatization procedures see Materials and methods. Molar ratio of ligand
to protein given in parentheses.
Chemical structure Abbreviation
Amount of ligand
(nmolÆmg
)1
)
aKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P Kdo4PGlcN
2
P
2
aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P Kdo5PGlcN
2
P
2
aKdo-4/5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P-BSA LPS
deac
-BSA 33 (2.4)
aKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P-BSA Kdo4P-GlcN
2
P
2
-BSA 16 (1.1)
aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P-BSA Kdo5P-GlcN
2
P
2
-BSA 15 (1.0)
1238 S. Mu
¨ller-Loennies et al. (Eur. J. Biochem. 269)ÓFEBS 2002
1 h with peroxidase-conjugated goat anti-(mouse IgG) Ig or
goat anti-(rabbit IgG) Ig (heavy and light chain specific,
Dianova; diluted 1 : 1000 in blocking buffer, 50 lL). Further
development of the reaction was as described above. All tests
were set up in quadruplicate. Confidence values of the means
were less than 10%.
RESULTS
Isolation and structural analysis of the phosphorylated
carbohydrate backbone of I-69 LPS
The LPS of H. influenzae I-69 was successively de-O-acylated
and de-N-acylated with hydrazine and potassium hydrox-
ide, respectively, leading to two major products as revealed
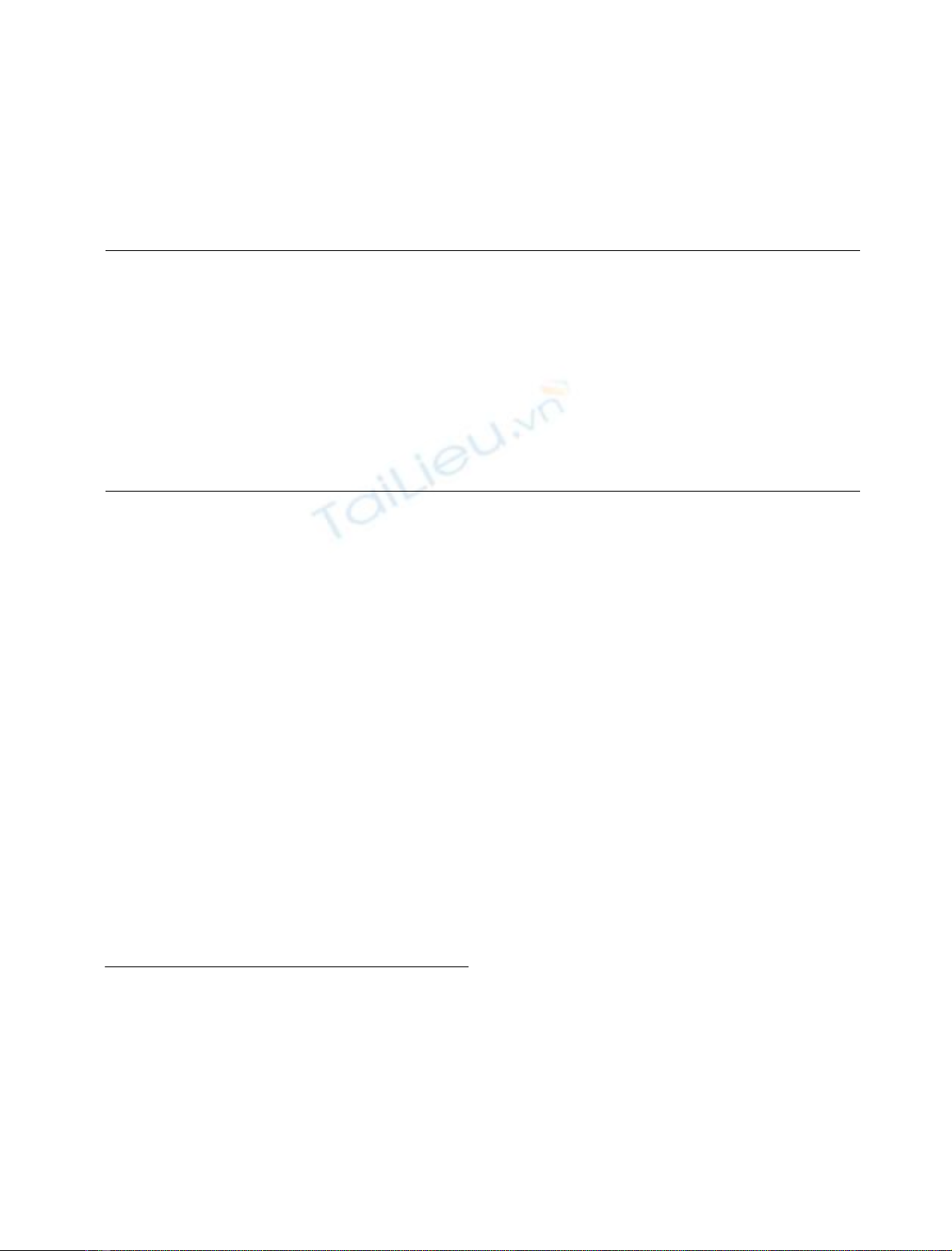
by HPAEC (Fig. 1). The two peaks, compounds 1 and 2,
could be separated from each other by preparative HPAEC
with yields of 11.6 mg (21.6% of LPS) and 5.1 mg (9.5%
of LPS) for Kdo-4P-GlcN
2
-P
2
and Kdo-5P-GlcN
2
-P
2
,
respectively.
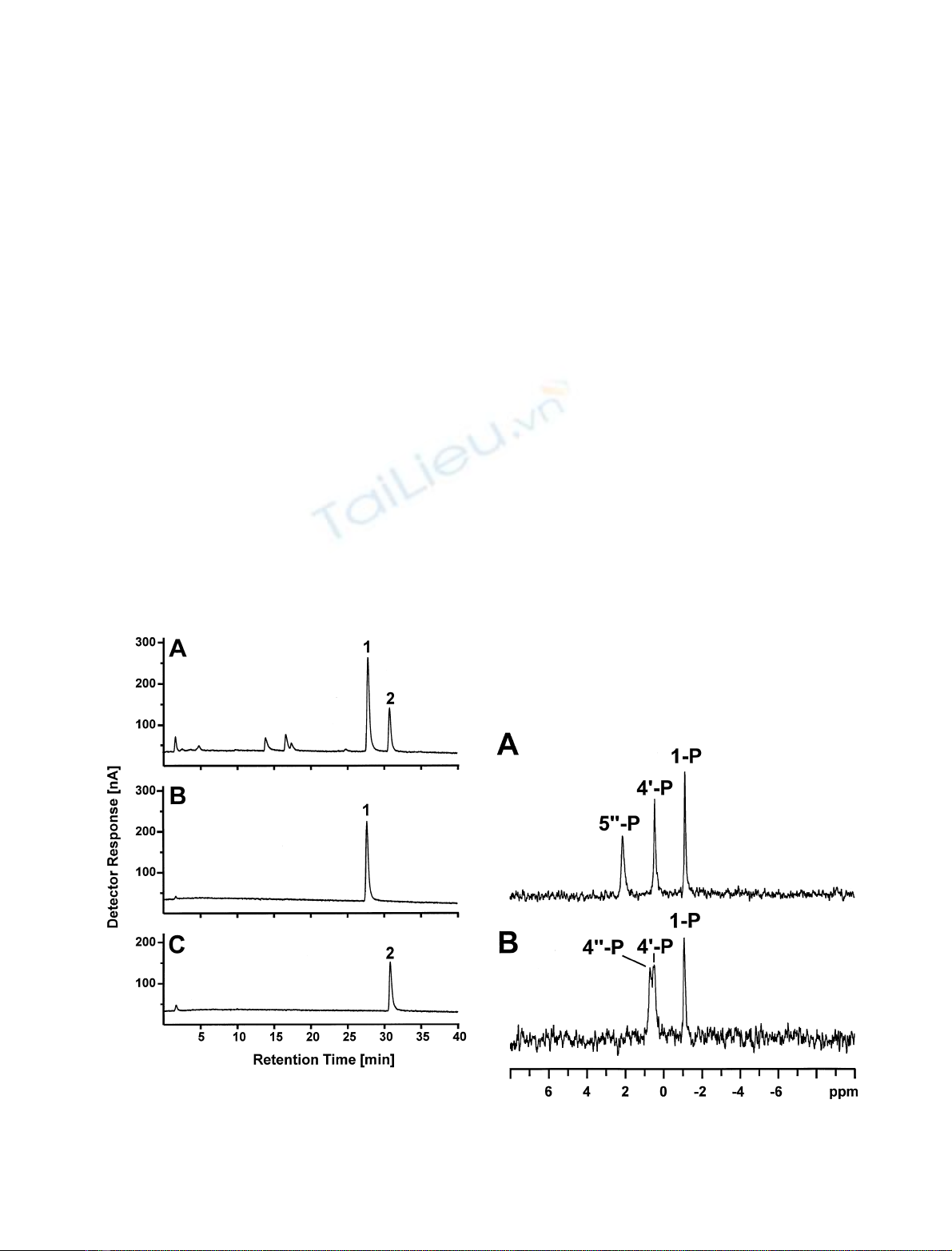
Both compounds were identified by one- and two-
dimensional NMR spectroscopy. Spectra of both contained
characteristic signals of a single a-Kdo-residue, one b-linked
GlcN and one a-configured GlcN [7]. In addition, three
phosphate-residues were identified by
31
P-NMR spectro-
scopy (Fig. 2). With respect to the carbohydrate and
phosphate composition the two compounds were identical
and was reflected by almost identical one-dimensional
1
H-NMR spectra (Fig. 3, Table 2). As expected the com-
pounds differed in their phosphate substitution (Fig. 3,
Table 4). Both compounds contained one glycosidic phos-
phate linked to the a-GlcN (A) of the lipid A backbone
leading to a splitting of the signal of its anomeric proton and
another phosphate linked to the 4-position of the b-config-
ured GlcN (B). The far downfield position of the chemical
shifts of proton H-4 and carbon C-4 of the Kdo-residue (C)
of compound 1 and the downfield shift to the same
frequencies of proton H-5 and carbon C-5 of the Kdo-resi-
due (C) of compound 2 identified compound 1 as Kdo-4P-
GlcN
2
-P
2
and compound 2 as Kdo-5P-GlcN
2
-P
2
(Tables 2–4). The correct position of phosphates was finally
determined by
1
H,
31
P-HMQC spectroscopy.
Serology
Both oligosaccharides were activated with glutardialdehyde
and conjugated to BSA as described [12]. Chemical analyses
indicated a molar ratio of protein to ligand of 1 : 1.1 and
1 : 1.0 for Kdo-4P-GlcN
2
-P
2
-BSA and Kdo-5P-GlcN
2
-
P
2
-BSA, respectively. Both neoglycoconjugates were used in
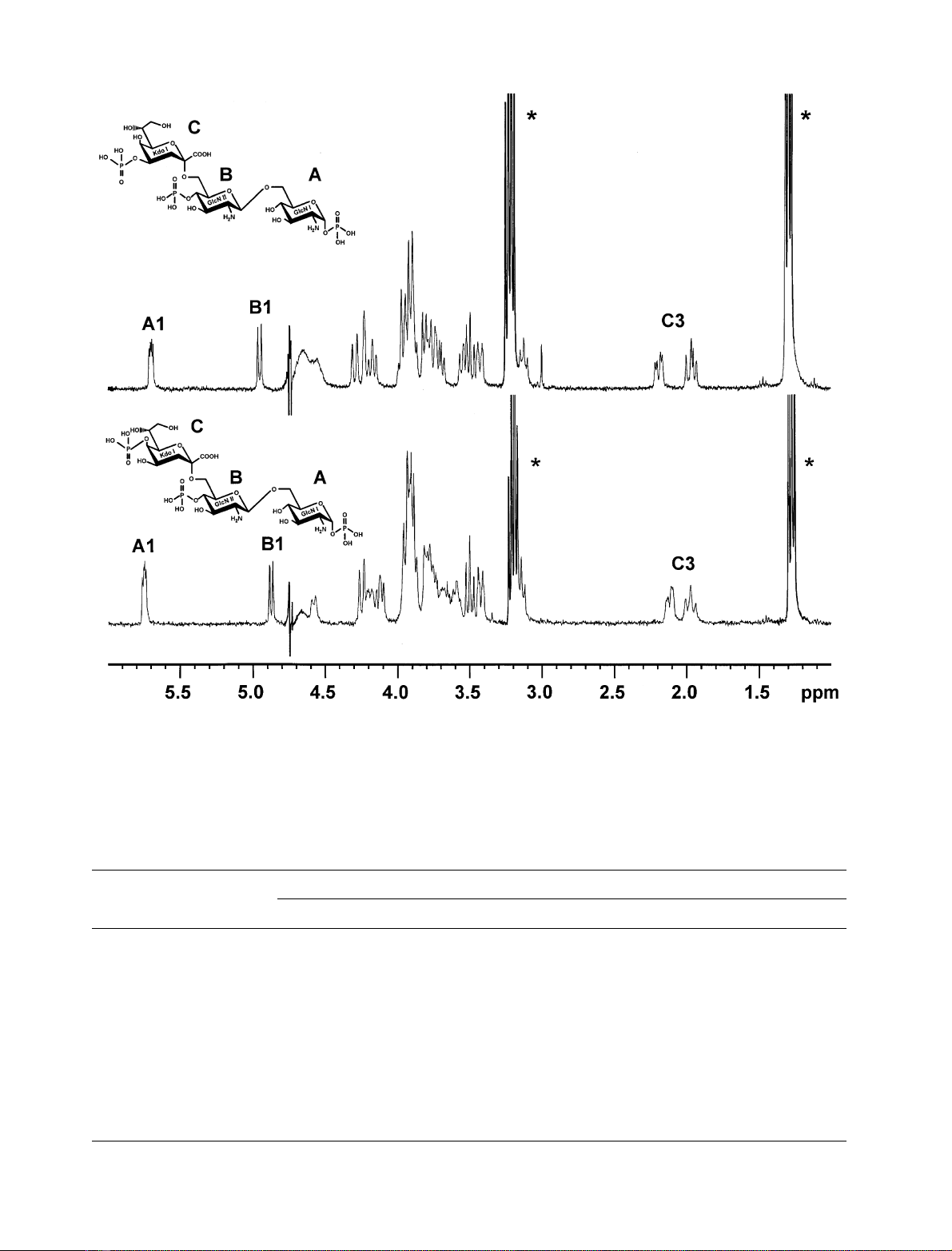
ELISA to determine the epitope specificities of mAb. LPS
and LPS
deac
-BSA were used for comparison, whereby the
latter contained a mixture of 4- and 5-phosphorylated Kdo
in the ratio as it occurs in natural LPS. Clone S42-16 and
S42-21 were confirmed to be specific for Kdo-5Pand
Kdo-4P, respectively. As seen in Fig. 4B clone S42-16
bound over a wide range of antigen coating concentrations
(10–0.08 pmol per well) to Kdo-5P-GlcN
2
-P
2
-BSA at
antibody concentrations as low as 1 ngÆmL
)1
. No binding
of this antibody was observed with Kdo-4P-GlcN
2
-P
2
-BSA
even at highest antigen concentration (10 pmol per well)
and antibody concentration (10 lgÆ mL
)1
) (Fig. 4A). The
mAb S42-21 bound only to Kdo-4P-GlcN
2
-P
2
-BSA
(Fig. 4C) but not to Kdo-5P-GlcN
2
-P
2
-BSA (Fig. 4D)
The affinity of mAb S42-21 was approximately 200 times
lower than that of mAb S42-16 for the homologous epitope.
Fig. 1. HPAEC chromatogram of deacylated LPS from H. influenzae
I-69. Shown is the analytical separation of the crude mixture (A) and
the analytical chromatography of the isolated species (B and C). Peaks
1and2representaKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P
and aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P, respectively.
Fig. 2.
31
P-NMR spectrum of aKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-
aGlcN-1P(top) and aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P
(bottom).
ÓFEBS 2002 Structure and antigenicity of H. influenzae LPS (Eur. J. Biochem. 269) 1239
The generation of clone S42-10-8 has been reported
previously [8] but its epitope specificity could not be
determined so far. Binding of this antibody was tested in
ELISA using various concentrations of Kdo-4P-GlcN
2
-
P
2
-BSA and Kdo-5P-GlcN
2
-P
2
-BSA, LPS or LPS
deac
-
BSA.
Fig. 3.
1
H-NMR spectra of aKdo-4P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P(top) and aKdo-5P-(2 fi6)-bGlcN-4P-(1 fi6)-aGlcN-1P(bottom).
The asterisk indicates signals of tryethylamine.
Table 2.
1
H-NMR chemical shift data of compounds 1 and 2. NR, not resolved.
Compound Residue
1
H-Chemical shift (p.p.m.) and coupling constants (Hz) for proton
H-1 H-2 H-3ax H-3eq H-4 H-5 H-6a H-6b H-7 H-8a H-8b
1Afi6aGlcN1P5.659 3.380 3.873 3.448 4.123 3.852 4.248
4; 8
a
10 10 10 12; 9 4
Bfi6bGlcN4P4.908 3.072 3.859 3.859 3.740 3.495 3.698
810
CaKdo4P1.925 2.149 4.514 4.179 3.766 3.926 3.918 3.652
)12; 12 6 6 9 12; NR 6
2Afi6aGlcN1P5.654 3.364 3.889 3.426 4.124 3.861 4.229
4; 7
a
11 10 10 12; 9 4
Bfi6bGlcN4P4.902 3.074 3.854 3.840 3.727 3.471 3.714
81010
CaKdo5P1.919
)12; 12
2.142
5
4.141 4.507 3.736
9
3.907 3.941
13; NR
3.649
a3
J
H-1,P
.
1240 S. Mu
¨ller-Loennies et al. (Eur. J. Biochem. 269)ÓFEBS 2002
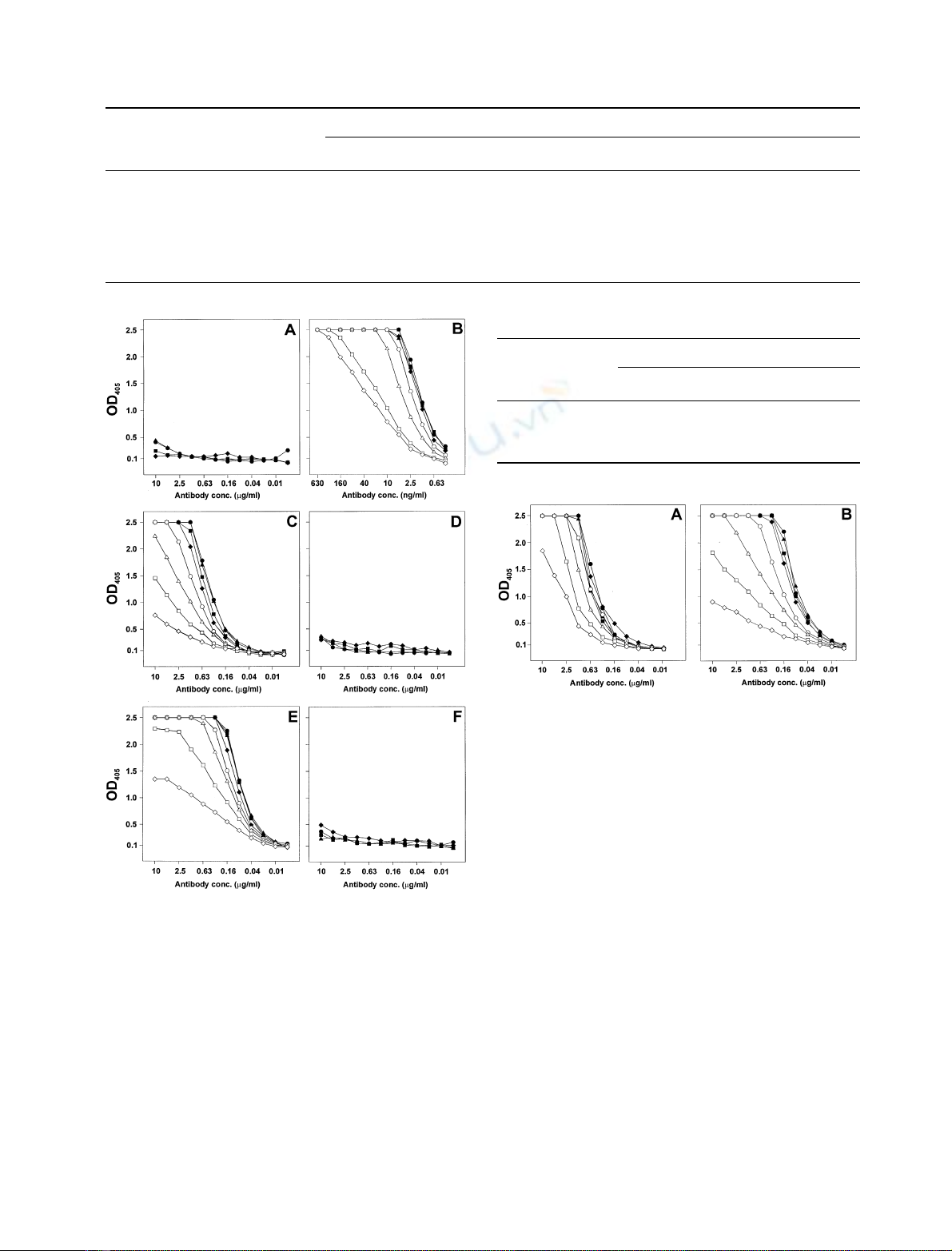
As seen in Fig. 4E, mAb S42-10-8 bound to Kdo-4P-
GlcN
2
-P
2
-BSA and with comparable affinity to LPS
(Fig. 5A) or LPS
deac
-BSA (Fig. 5B) as solid phase antigen.
No binding was observed with Kdo-5P-GlcN
2
-P
2
-BSA
(Fig. 4F).
The data show, together with those published earlier [8],
that mAb S42-10-8 binds to a complex epitope composed of
Kdo-4Plinked to the bisphosphorylated glucosamine
backbone of the LPS of H. influenzae I-69.
Although, Kdo-4Palone is not bound to the antibody,
the position of the phosphate group strictly determines the
specificity of the epitope as no binding was observed with
antigens containing Kdo-5Pinstead of Kdo-4Por with
antigens containing nonphosphorylated Kdo.
DISCUSSION
Kdo is a common constituent of LPS and its presence is
essential for the survival of Gram-negative bacteria. Ac-
cording to our present knowledge of the Kdo-lipid A region
one Kdo residue is linked to position 6¢of the glucosamine
disaccharide backbone of lipid A and is substituted at
position 5 by another sugar and at position 4 by another
sugar or phosphate [13]. The LPS of the deep rough mutant
I-69 of H. influenzae is unique in being composed of only one
Table 4.
31
P-NMR chemical shifts of compounds 1 and 2.
Residue
31
P-Chemical shift (p.p.m.) for compound
12
A)1.41 )1.64
B 0.58 )0.01
C 0.68 1.65
Table 3.
13
C-NMR chemical shift data of compounds 1 and 2. ND, not determined.
Compound Residue
13
C-Chemical shift ( p.p.m.) of carbon
C-1 C-2 C-3 C-4 C-5 C-6 C-7 C-8
1Afi6aGlcN1P91.10 54.45 69.76 70.07 72.69 69.36
Bfi6bGlcN4P99.14 55.74 72.08 74.44 74.20 62.40
CaKdo4PND ND 33.77 70.98 65.88 71.68 69.77 63.47
2Afi6aGlcN1P91.16 54.45 69.61 70.12 72.68 69.20
Bfi6bGlcN4P99.02 55.76 72.02 74.52 74.09 62.60
CaKdo5PND ND 34.92 65.99 71.00 71.64 69.65 63.07
Fig. 4. Binding curves of mAbs S42-16 (A and B), S42-21 (C and D),
and S42-10-8 (E and F) to Kdo4P-GlcN
2
P
2
-BSA (A, C and E) and
Kdo5P-GlcN
2
P
2
-BSA (B, D and F). ELISA plates were coated with 200
(d), 100 (m), 50 (j)25(r), 12.5 (s), 6.3 (n), 3.2 (h)and1.6(e)
pmol ligandÆml
)1
and reacted with the mAb concentrations indicated
on the abscissa. Values are the mean of quadruplicates with confidence
values not exceeding 10%.
Fig. 5. Binding curve of mAb S42-10-8. The ligands were I-69 LPS (A)
and LPS
deac
-BSA (B). The coating concentrations used were 400 (d),
200 (m), 100 (j)50(r), 25 (s), 12.5 (n), 6.3 (h)and3.2(e)
pmolÆml
)1
for LPS
deac
-BSA. Due to the poor coating efficiency of LPS
2000 (d), 1000 (m), 500 (j)250(r), 125 (s), 63 (n), 32 (h)and16
(e)pmolÆml
)1
were used for the immobilization of LPS. Both were
reacted with mAb concentrations indicated on the abscissa. Values are
the mean of quadruplicates with confidence values not exceeding 10%.
ÓFEBS 2002 Structure and antigenicity of H. influenzae LPS (Eur. J. Biochem. 269) 1241



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





