
Characterization of a partially folded intermediate of stem
bromelain at low pH
Soghra Khatun Haq, Sheeba Rasheedi and Rizwan Hasan Khan
Interdisciplinary Biotechnology Unit, Aligarh Muslim University, India
Equilibrium studies on the acid included denaturation of
stem bromelain (EC 3.4.22.32) were performed by CD
spectroscopy, ¯uorescence emission spectroscopy and
binding of the hydrophobic dye, 1-anilino 8-naphthalene
sulfonic acid (ANS). At pH 2.0, stem bromelain lacks a well
de®ned tertiary structure as seen by ¯uorescence and near-
UV CD spectra. Far-UV CD spectra show retention of some
native like secondary structure at pH 2.0. The mean residue
ellipticities at 208 nm plotted against pH showed a transition
around pH 4.5 with loss of secondary structure leading to
the formation of an acid-unfolded state. With further
decrease in pH, this unfolded state regains most of its sec-
ondary structure. At pH 2.0, stem bromelain exists as a
partially folded intermediate containing about 42.2% of the
native state secondary structure Enhanced binding of ANS
was observed in this state compared to the native folded state
at neutral pH or completely unfolded state in the presence of
6
M
GdnHCl indicating the exposure of hydrophobic regions
on the protein molecule. Acrylamide quenching of the
intrinsic tryptophan residues in the protein molecule showed
that at pH 2.0 the protein is in an unfolded conformation
with more tryptophan residues exposed to the solvent as
compared to the native conformation at neutral pH. Inter-
estingly, stem bromelain at pH 0.8 exhibits some charac-
teristics of a molten globule, such as an enhanced ability to
bind the ¯uorescent probe as well as considerable retention
of secondary structure. All the above data taken together
suggest the existence of a partially folded intermediate state
under low pH conditions.
Keywords: acid denaturation; circular dichroism; partially
folded intermediate; stem bromelain.
The molecular mechanism of the spontaneous folding of
proteins from a random polypeptide chain to the well
ordered native conformation is still unknown. Results of
kinetic refolding experiments in vitro as well as theoretical
considerations suggest that folding of large proteins is a
sequential hierarchical process [1]. Various proteins have
been observed to exist in stable conformations that are
neither fully folded nor unfolded and are said to be in the
Ômolten globuleÕstate [2]. These partially folded intermedi-
ates can be made to accumulate in equilibrium by mild
concentrations of chemical denaturants, low pH, covalent
trapping or by protein engineering [3]. It is now generally
accepted that protein folding involves a discrete pathway
with intermediate states between native and denatured states
[4]. A number of globular proteins are known to show the
equilibrium unfolding transition that does not obey the two-
state rule but exhibits a compact intermediate that has an
appreciable amount of secondary structure [5±8]. Acid-
induced unfolding of proteins is often incomplete and the
acid-unfolded proteins assume conformations that are
different from the fully unfolded ones observed in the
presence of 6
M
GdnHCl or 9
M
urea [9±11]. Such stable
conformational states located between the native and
unfolded states have been found for several proteins [12].
Several studies have shown that the compactness and the
amount of secondary structure of the intermediate states
formed in the folding pathway of proteins are not neces-
sarily close to those of the native state, but vary greatly
depending on the protein species [1,13]. This suggests the
presence of various intermediate states, from one close to
the fully unfolded state to one close to the native state
depending upon the protein and the experimental condi-
tions [14].
The characteristic features of a Ômolten-globuleÕare: (a) it
is less compact than the native state; (b) it is more compact
than the unfolded state; (c) it contains extensive secondary
stricture; and (d) it has loose tertiary contacts without tight
side-chain packing. Recently, increasing evidence supports
the idea that the molten globule may possess well-de®ned
tertiary contacts [15±18]. Proteins in the molten globule state
contain high level of secondary structure, as well as a
rudimentary, native like tertiary topology. Thus, the struc-
tural similarity between the molten globule and native
proteins may have a signi®cant bearing in understanding the
protein-folding problem [19].
While a detailed study on the denaturation and refolding
aspects of papain, a thiol protease has been made by several
workers; no studies on the acid denaturation of stem
bromelain, a protelytic cysteinyl protease from Ananas
comosus has been made till date. Arroyo-Reyna et al. have
proposed that bromelain forms may have the same folding
pattern shown by other members of the papain family as the
spectral characteristics displayed by stem bromelain are
similar to those observed in case of papain and proteinase W
namely, a bilobal structure with predominantly aand
Correspondence to R. Hasan Khan, Interdisciplinary Biotechnology
Unit, Aligarh Muslim University, Aligarh 202002, India.
Fax: + 91 571 701081, Tel.: + 91 571 701718,
E-mail: rizwanhkhan@hotmail.com
Abbreviations: ANS, 1-anilino 8-naphthalene sulfonic acid.
Enzymes: stem bromelain (EC 3.4.22.32).
(Received 25 June 2001, revised 17 October 2001, accepted 19 October
2001)
Eur. J. Biochem. 269, 47±52 (2002) ÓFEBS 2002

antiparallel bsheet domains [20,21]. Stem bromelain
belongs to the a+bprotein class as other cysteine
proteinases do and the highly identical amino-acid sequenc-
es of papain [22], actinidin [23], proteinase W[24,25]
chymopapain [26,27] and stem bromelain [28] indicate that
the polypeptide chains of these proteins share a common
folding pattern. This has been con®rmed for the ®rst three
proteinases by detailed X-ray diffraction studies [21,29,30].
In the present communication, we demonstrate the presence
of a partially folded intermediate at pH 2.0 having disor-
dered side chain interactions but with considerable second-
ary structure and relatively more exposed hydrophobic
surface as seen by ¯uorescence, CD and ANS binding.
MATERIALS AND METHODS
Materials
Bromelain (EC 3.4.22.32) lot no. B4882 and 1-anilino
8-naphthalene sulfonic acid (ANS) were purchased from
Sigma Chemical Co., USA. Guanidine hydrochloride
(GdnHCl) was obtained from Qualigens, India. Acrylamide
and urea were purchased from Sisco Research Laboratories,
India. All other reagents were of analytical grade.
Autolysis inhibition
To avoid complications due to autocatalysis, enzyme
samples were irreversibly inactivated by the method of
Sharpira & Arnon [31] with certain modi®cations. Reduc-
tion was carried out in 0.32
M
2-mercaptoethanol for 4 h at
room temperature, followed by addition of solid iodoace-
tamide to give a ®nal concentration of 0.043
M
.After
stirring for 30 min at 4 °C, the solutions were dialyzed
overnight against 10 m
M
sodium phosphate buffer, pH 7.0.
This inactive derivative was used throughout the present
study.
Spectrophotometric measurements
The protein concentration was determined on a Hitachi
U-1500 Spectrophotometer using an extinction coef®cient
e1%
1cm;280nm 20.1 [32]. The molecular mass of the protein
was taken as 23 800 [33]. A stock solution of ANS in
distilled water was prepared and concentration determined
using an extinction coef®cient of e
M
5000
M
)1
cm
)1
at
350 nm [34]. The molar ratio of protein to ANS was 1 : 50.
Acid denaturation
Acid-induced unfolding of stem bromelain was carried out
in 10 m
M
solutions of the following buffers: glycine/HCl
(pH 0.8±2.2), sodium acetate (pH 2.5±6.0), sodium phos-
phate (pH 7.0±8.0) and glycine/NaOH (pH 9.0±10.0). pH
measurements were carried out on an Elico digital pH
meter (model LI 610) with a least count of 0.01 pH unit.
Stem bromelain (12.6±37.8 l
M
) was incubated with the
buffers of desired pH at 4 °C and allowed to equilibrate for
4 h before taking the spectrophotometric measurements. In
order to assess the reversibility of acid induced unfolding,
stem bromelain at pH 2.0 was extensively dialyzed against
10 m
M
sodium phosphate buffer, pH 7.0. This dialyzed
preparation was compared to stem bromelain at pH 7.0 and
the partially folded state at pH 2.0 using ¯uorescence and
CD.
Fluorescence measurements
Fluorescence measurements were carried out on a Shimadzu
Spectro¯uorometer (model RF-540) equipped with a data
recorder DR-3 and on a Hitachi Spectro¯urometer (model
F-2000). The concentration of stem bromelain used was in
the range 13.9±14.5 l
M
. For the intrinsic tryptophan
¯uorescence, the excitation wavelength was set at 280 nm
and the emission spectra recorded in the range of 300±
400 nm with 5- and 10-nm slit widths for excitation and
emission, respectively. Binding of ANS to stem bromelain at
various pH values was studied by exciting the dye at 380 nm
and the emission spectra were recorded from 400 to 600 nm
with 10-nm slit width for excitation and emission.
CD measurements
CD measurements were carried out on a Jasco J-720
Spectropolarimeter equipped with a microcomputer and
precalibrated with (+)-10-camphorsulfonic acid. All the
CD measurements were carried out at 30 °C and each
spectrum was recorded as an average of two scans. The
near-UV spectra were recorded in the wavelength region of
250±300 nm with a protein concentration of 0.9 mgmL
)1
in a 10-mm pathlength cuvette. The far-UV CD studies were
made in the wavelength region of 200±250 nm with a
concentration of 0.3 mgmL
)1
in a 1-mm pathlength
cuvette.
GdnHCl induced denaturation
Denaturation of stem bromelain at pH 2.0 in the presence
of guanidine hydrochloride was studied by far-UV CD.
Increasing amounts of 7.2
M
GdnHCl were added to a ®xed
concentration (21 l
M
) of protein and allowed to equilibrate
before taking CD measurements at 222 nm. Mean residue
ellipticity (MRE) values were calculated according to Chen
et al. [35] and plotted against denaturant concentration.
Fraction of protein denatured ( f
D
) was calculated according
to Tayyab et al.[36].
Acrylamide quenching
Quenching of intrinsic tryptophan ¯uorescence was per-
formed on a Hitachi Spectro¯uorometer (model F-2000)
using a stock solution of 5
M
acrylamide. To a ®xed amount
(17.2 l
M
) of protein, increasing amounts of acrylamide
(0.1±1.0
M
) were added and the samples incubated for
30 min prior to taking the ¯uorescence measurements. For
the intrinsic tryptophan ¯uorescence spectra, the protein
samples were excited at 295 nm and emission spectra
recorded between 250 and 550 nm and the data obtained
were analyzed according to the Stern±Volmer equation [37].
RESULTS AND DISCUSSION
The acid denaturation of stem bromelain was studied over a
pH range of 0.8±10.0. Stem bromelain contains ®ve
tryptophan residues [28] and extensive sequence homology
with papain suggests that three tryptophans are buried in
48 S. Khatun Haq et al. (Eur. J. Biochem. 269)ÓFEBS 2002
hydrophobic core whereas two of them are located near the
surface of the molecule. As the intrinsic ¯uorophore
tryptophan is highly sensitive to the polarity of its
surrounding environment, the pH dependent changes in
the conformation of stem bromelain were followed using
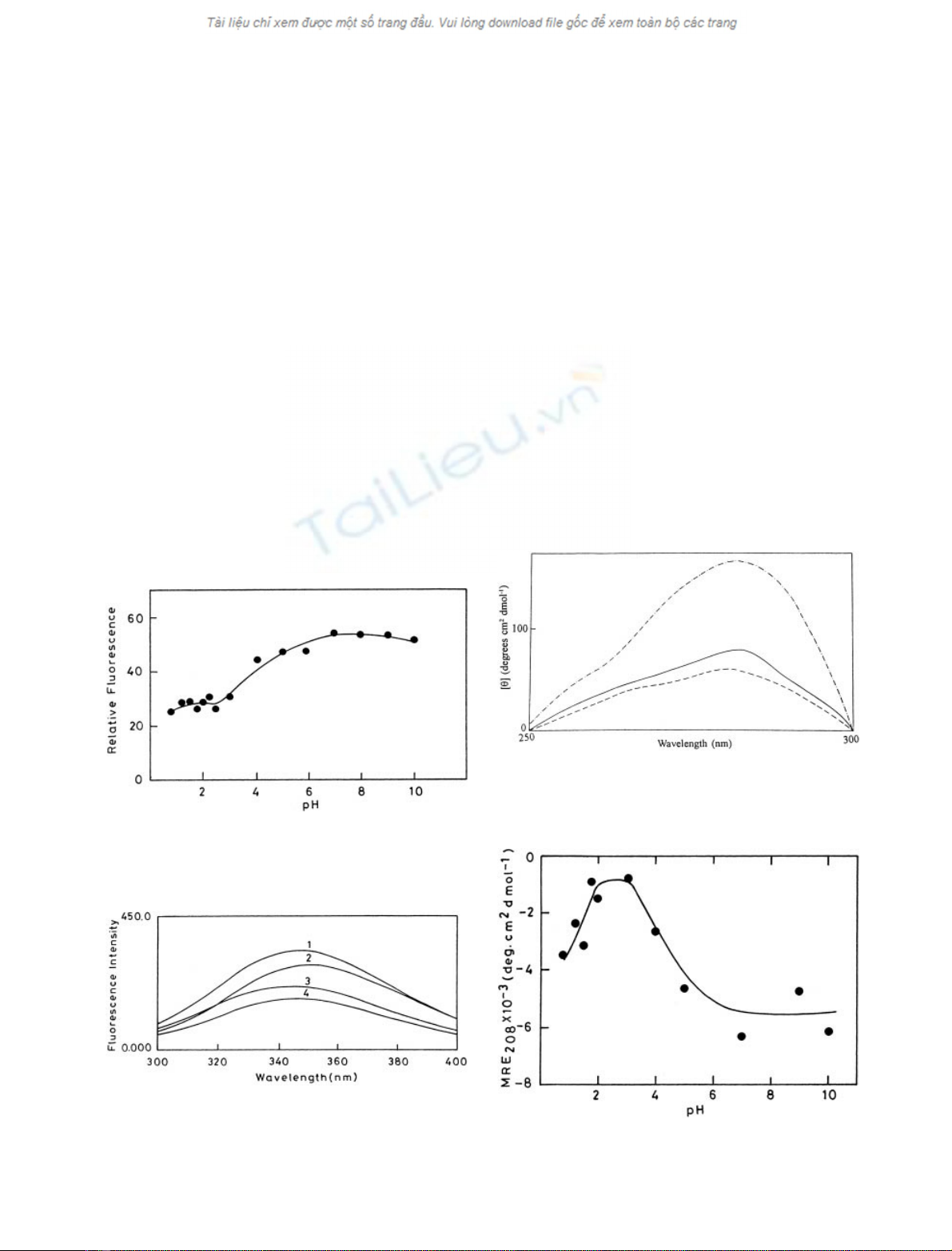
¯uorescence spectroscopy. As seen from Fig. 1, with the
lowering of pH, the relative ¯uorescence of stem bromelain
gradually decreases to pH 2.0 and becomes more or less
constant, indicative of the presence of a non-native stable
intermediate at low pH.
The emission spectrum of stem bromelain at pH 7.0
(Fig. 2) shows a maximum at 347 nm that suggests that
some of the tryptophan residues of the protein are relatively
more exposed to solvent. However at pH 2.0 there is a
decrease in the ¯uorescence emission intensity with a slight
blue shift (3±4 nm). This blue-shifted ¯uorescence of stem
bromelain at pH 2.0 can be attributed to the conforma-
tional changes in the vicinity of the surface exposed
tryptophans; in this case internalization in a hydrophobic
environment. A similar blue-shifted ¯uoresence has been
reported earlier for glucose isomerase [37], bovine growth
hormone [38] and interferon-c[39]. The addition of 2
M
urea
to the protein at pH 2.0 further decreases the ¯uorescence
intensity apparently without altering the microenvironment
of the aromatic ¯uorophore. The completely unfolded state
of bromelain in the presence of 6
M
GdnHCl shows a red
shift of 4 nm with a concomitant decrease in the ¯uores-
cence intensity. These observations suggest that the protein
at pH 2.0 is present in a conformational state that is
different from the native state at pH 7.0 as well as
completely unfolded state in the presence of 6
M
GdnHCl.
Figure 3 shows the near UV CD spectra of the native
state of the protein, the denatured state of the protein and of
the acid-induced state at pH 2.0. As seen in the ®gure, the
spectrum of stem bromelain at pH 2.0 differs from that at
pH 7.0 and resembles the denatured state of the protein in
presence of 6
M
GdnHCl. This suggests that the protein at
pH 2.0 has most of its tertiary contacts disrupted. However,
the presence of loose tertiary interactions in the absence of
tight side chain packing cannot be ruled out.
The changes in the secondary structure of stem bromelain
as a function of pH were also followed by far-UV CD by
measuring mean residue ellipticity values at 208 nm (Fig. 4).
A cooperative transition from the native to the unfolded
state occurs in the vicinity of pH 4.5 re¯ecting loss of
secondary structure. However, at pH 2.0, stem bromelain
retains some secondary structural features (Fig. 5). On
further lowering of pH; stem bromelain regains a signi®cant
amount (42.2%) of the lost secondary structure due to
effective shielding of repulsive forces by the anions but the
tertiary structural loss as seen by near-UV CD is not
regained.
Fig. 1. Eect of pH on the emission ¯uoresence intensity of stem
bromelain. Ten millimolar solutions of glycine/citrate/phosphate buf-
fers were used in the pH range 0.8±10.0.
Fig. 2. Spectroscopic characterization of stem bromelain: ¯uoresence
emission spectra of stem bromelain at pH 7.0 (1), pH 7.0 + 6
M
GdnHCl (2), pH 2.0 (3) and pH 2.0 + 2
M
urea (4). Excitation and
emission wavelengths were 280 nm and 345 nm, respectively.
Fig. 3. Near UV-CD spectra of stem bromelain. Native protein at
pH 7.0 (ÐÐ), acid-induced state at pH 2.0 (Ð) and 6
M
GdnHCl
denatured state (± ±).
Fig. 4. Eect of pH on the mean residue ellipticity (MRE) of stem
bromelain. Ellipticity was monitored at 208 nm by far UV CD.
ÓFEBS 2002 Partially folded intermediate of stem bromelain (Eur. J. Biochem. 269)49



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





