
The oxidative effect of bacterial lipopolysaccharide on native and
cross-linked human hemoglobin as a function of the structure
of the lipopolysaccharide
A comparison of the effects of smooth and rough lipopolysaccharide
Douglas L. Currell and Jack Levin
Department of Laboratory Medicine, University of California School of Medicine and Veterans Administration Medical Center,
San Francisco, CA, USA
The binding of lipopolysaccharide (LPS, also known as
bacterial endotoxin) to human hemoglobin is known to
result in oxidation of hemoglobin to methemoglobin and
hemichrome. We have investigated the effects of the LPSs
from smooth and rough Escherichia coli and Salmonella
minnesota on the rate of oxidation of native oxyhemoglobin
A
0
and hemoglobin cross-linked between the a-99 lysines.
For cross-linked hemoglobin, both smooth LPSs produced a
rate of oxidation faster than the corresponding rough LPSs,
indicating the importance of the binding of LPS to the
hemoglobin. The effect of the LPS appeared to be largely on
the initial fast phase of the oxidation reaction, suggest-
ing modification of the heme pocket of the achains. For
hemoglobin A
0,
the rates of oxidation produced by rough
and smooth LPSs were very similar, suggesting the possibility
that the effect of the LPSs was to cause dissociation of
hemoglobin into dimers. The participation of cupric ion in
the oxidation process was demonstrated in most cases. In
contrast, the rate of oxidation of cross-linked hemoglobin by
the LPSs of both the rough and smooth E. coli was not
affected by the presence of chelators, suggesting that cupric
ion had previously bound to these LPSs. Overall, these data
suggest that the physiological effectiveness of hemoglobin
solutions now being developed for clinical use may be
decreased by the presence of lipopolysaccharide in the
circulation of recipients.
Keywords: bacterial endotoxin (lipopolysaccharide); human
hemoglobin; oxidation of hemoglobin; cross-linked hemo-
globin.
The interaction between bacterial lipopolysaccharide (LPS,
also known as bacterial endotoxin) and human hemoglobin
(Hb) has been shown in previous studies to affect the
properties of both the Hb molecule and the LPS [1–3]. The
binding of Hb to the smooth LPSs, Escherichia coli 026:B6
and Proteus mirabilis S 1959, was demonstrated and shown
to cause disaggregation and an increase of the biological
activity of the LPS [1]. In a related study, Hb similarly
enhanced activation of Limulus amebocyte lysate and
stimulation of endothelial cell tissue factor production by
smooth or rough P. mirabilis [2]. Rough LPS lacks the
polysaccharide side-chain that is present in the complete
(smooth) LPS molecule. In contrast, Limulus amebocyte
lysate activation either by lipid A (which consists of a
phosphorylated disaccharide backbone with several long-
chain fatty acids) or partially deacylated Salmonella minne-
sota 595 (Re) LPS was not enhanced in the presence of Hb.
The effect of Hb on the LPS and purified lipid A of rough
E. coli has been recently investigated, and significant
physical changes in the purified lipid A and in the lipid A
moiety of intact LPS were reported [4].
The binding of LPS to oxyHb results in the oxidation of
theHbtometHbandhemichrome[3].Incontrasttothe
lack of effect of Hb on the biological activity of partially
deacylated LPS from S. minnesota 595, this LPS was more
effective in producing oxidation of Hb than the LPS of
either rough S. minnesota 595 or smooth P. mirabilis [3]. To
further clarify these structure–function relationships, we
have extended these studies to compare the effects of
smooth and rough LPSs of E. coli and S. minnesota on the
oxidation of native and cross-linked Hb. Because the auto-
oxidationofHbhasbeenshowntodependonthepHand
the presence of heavy metal cations [5–8], we have also
investigated the effects of pH, EDTA and neocuproine on
the LPS-mediated oxidation of Hb.
MATERIALS AND METHODS
Bacterial lipopolysaccharides
Smooth E. coli lipopolysaccharide 026:B6 (Westphal
method [9]) was obtained from Difco Laboratories (Detroit,
MI, USA). Rough E. coli J5 (Rc) and smooth S. minnesota
(Galanos method [10]) were generously provided by
K. Meyers (RIBI Immunochem Research, Inc., Hamilton,
MT, USA). Deep rough S. minnesota 595 (Re) lipopoly-
saccharide (Westphal method [9]) was obtained from List
Biological Laboratories, Inc. (Campbell, CA, USA).
The lipopolysaccharides (5.0–5.9 mg) were suspended in
l.0 mL NaCl/P
i
(0.9% NaCl), pH 7.4, by treatment for
Correspondence to J. Levin, V. A. Medical Center (111-H2) 4150
Clement Street, San Francisco, CA 94121, USA.
Fax: + 1 415 831 2506, Tel.: + 1 415 750 6913,
E-mail: levinj@medicine.ucsf.edu
Abbreviations: LPS, lipopolysaccharide; Hb, human hemoglobin.
(Received 19 April 2002, revised 11 July 2002, accepted 1 August 2002)
Eur. J. Biochem. 269, 4635–4640 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03163.x

5 min in an ultrasonic bath (Branson Ultrasonic Cleaner,
Shelton, CT, USA), after initial suspension with a vortex
mixer. The LPS suspensions were stored at 0–4 Cand
immediately before use were retreated with the vortex mixer.
Reagents
LPS-free NaCl/P
i
was obtained from Irvine Scientific (Santa
Ana, CA, USA) and was diluted with deionized water to
produce a buffer, pH 7.4, 0.1
M
phosphate and 0.15
M
NaCl. All other phosphate buffers used were prepared from
monobasic NaH
2
PO
4
(Fisher Scientific Co., Fairlawn, NJ,
USA) and dibasic K
2
HPO
4
(J. T. Baker Chemical Co.,
Phillipsburg, NJ, USA) and used as 0.2
M
solutions. Tricine
was obtained from Sigma Chemical Co. (St Louis, MO,
USA)andusedasa0.15
M
solution. Neocuproine and
EDTA were obtained from Sigma Chemical Co. and used
as a 0.01
M
aqueous solution and a 0.1
M
solution in NaCl/
P
i
, respectively.
Hemoglobin
Hemoglobin A
0
,58mgÆmL
)1
, in Ringer’s lactate, pH 8.0,
which had been purified by ion-exchange HPLC as
described previously [11], was provided by the Blood
Research Detachment, Walter Reed Army Institute of
Research, Washington, D.C., USA and stored at )70 C
until use. The initial metHb concentration of Hb A
0
was
always < 5%. Human Hb, cross-linked between the Lys99
residues of the achains by treatment of deoxyHb with
bis(3,5-dibromosalicyl) fumarate, also was provided by the
Blood Research Detachment [12]. The stock solution was
71 mgÆmL
)1
in Ringer’s acetate, pH 7.4. It was sterile and
essentially LPS-free (< 100 pgÆmL
)1
as assessed by the
Limulus amebocyte lysate assay [13]) and stored at )70 C
until use. The initial metHb concentration of the cross-
linked Hb was always < 7%.
Copper analysis
All reagents, buffers, Hb stock solutions and LPS suspen-
sions (containing 5.0–5.9 mgÆmL
)1
LPS) were analyzed for
cupric ion by M. Qian in the laboratory of J. W. Eaton,
James Graham Brown Cancer Center, University of
Louisville, Louisville, KY, USA, by the method of Makino
[14]. The results are presented in Table 1.
Oxidation experiments
To 360 lL of buffer was added 6.0 lL of a cross-linked Hb
solution, 71 mgÆmL
)1
,or7.0lLofaHbA
0
solution,
58 mgÆmL
)1
,andthen80lL of a suspension of LPS,
5.0–5.9 mgÆmL
)1
, to produce an LPS/Hb suspension of
approximately equal concentrations (mgÆmL
)1
): the final
Hb concentration was 0.8–1.0 mgÆmL
)1
.Insomeexperi-
ments, 4.4 lLEDTA,0.1
M
,or4.4 lL neocuproine, 0.01
M
,
was added. The absorption spectrum from 400 nm to
800 nm was measured at selected time intervals during a 2-h
period, using a Beckman DU-7400 spectrophotometer
(Beckman Instruments, Inc., Fullerton, CA, USA). All
experiments were carried out at 37 C. The temperature was
maintained by a circulation water bath, Lauda K-2/RD9
(Brinkman Instruments, Westbury, NY, USA). The neces-
sary correction for light scattering in all suspensions that
contained lipopolysaccharide was performed with a pro-
gram in the spectrophotometer software. The relative
concentrations of oxyHb, metHb and hemichromes were
obtained by the method of Winterbourn [15] from simul-
taneous measurements of absorbances at 560, 577 and
630 nm. The major oxidation product was metHb. The
amount of hemichrome produced during a 2-h reaction was
typically less than 10% (data not shown). The decrease in
concentration of oxyHb with time was utilized as a measure
of the rate of oxidation of oxyHb.
RESULTS
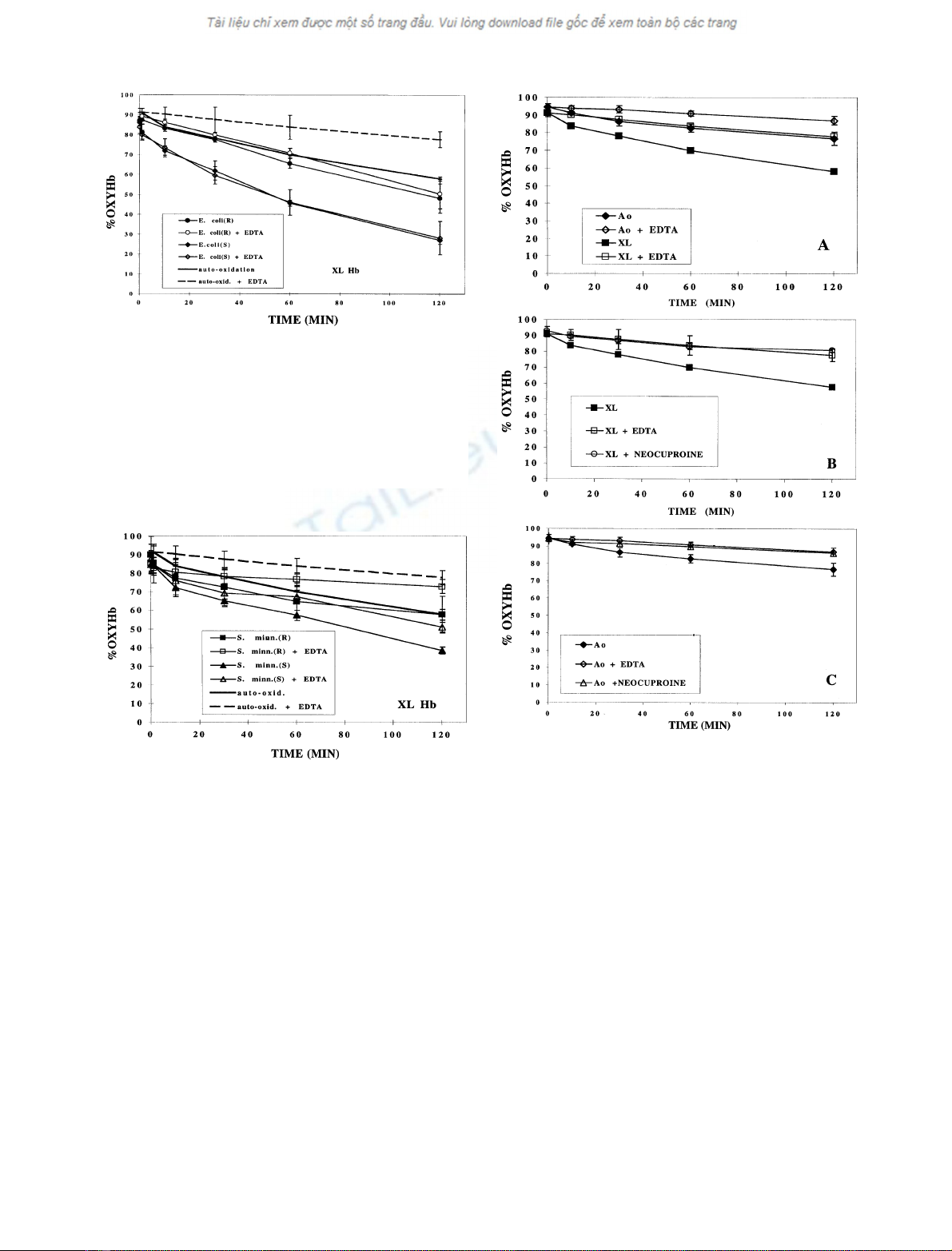
The effect of pH on the auto-oxidation of cross-linked Hb
was studied. The rate of decrease of the concentration of
oxyHb increased as the pH was lowered over the range from
pH 9.0–5.8 (data not shown). As was observed previously
by others [5], the reaction is biphasic at pH 7.4 and below,
with an initial fast phase followed by a slower phase. To
determine the optimum pH at which to study the effect of
LPS on the oxidation rate, a comparison of the effects of the
LPSs of smooth E. coli and rough S. minnesota on the
oxidation rate over the pH range 5.8–9.0 was undertaken
(data not shown). Because at pH 7.0 both LPSs produced
marked but distinguishable effects, all further experiments
were carried out at this pH.
The contribution of the polysaccharide component of
LPS to its effect on the oxidation of cross-linked Hb was
then investigated by a comparison of the effects of rough
andsmoothLPSsofE. coli, both in the presence and
absence of EDTA (Fig. 1). The rate of oxidation was
increased in the presence of the LPSs of both the smooth
and rough E. coli, but the rate of oxidation in the presence
of the LPS of smooth E. coli was much faster than for the
LPS from rough E. coli. Although EDTA markedly
decreased the rate of auto-oxidation, its effect on the
oxidation rate of cross-linked Hb in the presence of LPS was
negligible for both the smooth and rough LPSs (Fig. 1).
The effect of the LPSs of smooth and rough S. minnesota
on the oxidation rate of cross-linked Hb also was compared
(Fig. 2). The rate of oxidation in the presence of the LPS of
smooth S. minnesota was much faster than in the presence
Table 1. Copper concentration in Hb stock solutions, buffers and LPS
suspensions.
Sample Cu concentration (l
M
)
Hemoglobin
a,a-Hb (71 mgÆmL
)1
) 1.0
Hb A
0
(58 mgÆmL
)1
) 2.5
LPS (5.0–5.9 mgÆmL
)1
)
a
S. minnesota (R) 1.8
S. minnesota (S) 3.4
E. coli (R) 5.0
E. coli (S) 6.4
Buffers
Phosphate buffers, 0.2
M
0.8
Phosphate, 0.1
M
buffered-saline, 0.15
M
0.3
Tricine, 0.15
M
0.0
a
Cu concentrations are the mean of two determinations.
4636 D. L. Currell and J. Levin (Eur. J. Biochem. 269)FEBS 2002
of the LPS from rough S. minnesota, both in the presence
and absence of EDTA. In contrast to the results with the
LPSs of E. coli, EDTA decreased the rate of oxidation.
However, the rate of oxidation mediated by the smooth LPS
was less affected by the presence of EDTA. The rough
S. minnesota LPS increased the initial fast phase of the
reaction, but decreased the rate of the slow phase of
oxidation in the presence of EDTA.
A comparison of rough and smooth LPSs of E. coli and
S. minnesota in the presence of EDTA revealed that both in
the presence and absence of EDTA, the oxidation of cross-
linked Hb was faster in the presence of the smooth LPSs
(Figs 1 and 2). In addition, the rate of oxidation mediated
by the smooth E. coli LPS was faster than that produced by
the smooth S. minnesota LPS. The rate of oxidation in the
presence of the rough S. minnesota LPS was slower than
that produced by the other three LPSs studied.
A comparison of the auto-oxidation of cross-linked Hb
with that of Hb A
0
is shown in Fig. 3A. The effect of the
presence of EDTA, known to bind heavy metal cations [16],
on the oxidation of both Hbs is also presented in Fig. 3A.
The rate of auto-oxidation of cross-linked Hb was greater
than that of Hb A
0,
both in the presence and absence of
EDTA, as has been observed previously [17]. In addition, the
rates of auto-oxidation of both cross-linked Hb and Hb A
0
were markedly reduced by EDTA, suggesting catalysis of the
oxidation by heavy metal cations, as previously observed by
Rifkind [8,18]. To determine whether the heavy metal cation
was cupric ion as indicated by the results of Rifkind [8], the
effect of a chelator specific for cupric ion, neocuproine
[19,20], was studied. The results in Fig. 3B,C show that the
effects of neocuproine and EDTA on the oxidation rate were
identical, confirming that the cupric ion was the heavy metal
cation primarily responsible for the catalysis. The concen-
trations of cupric ion in the solutions used were determined
by chemical analysis (Table 1).
Fig. 1. Comparison of the effects of the LPSs of smooth E. coli 026:B6
and rough E. coli J5 (Rc), in the absence and presence of EDTA, on the
oxidation of a,a-cross-linked Hb (XL Hb). Hb concentration was
0.8 mgÆmL
)1
, in phosphate buffer, 0.2
M
,pH7.0.LPSconcentration
was 0.8–1.0 mgÆmL
)1
. The mean ± SD of three independent experi-
ments is shown. Each experiment was performed with aliquots of a
single sample of Hb. Therefore, apparent differences in the starting
oxyHb concentrations are the result of an immediate drop in the
oxyHb concentration upon addition of the LPS.
Fig. 2. Comparison of the effects of the LPSs of rough S. minnesota 595
(Re) and smooth S. minnesota, in the absence and presence of EDTA, on
the oxidation of a,a-cross-linked Hb. Themean±SDofthreeinde-
pendent experiments is shown. Other conditions as in Fig. 1.
Fig. 3. The effect of EDTA or neocuproine on the auto-oxidation of
a,a-cross-linked Hb (XL) and Hb A
0
(A
0
). Hb concentration was
0.8 mgÆmL
)1
, in phosphate buffer, 0.2
M
,pH7.0.Themean±SDof
three or four independent experiments is shown.
FEBS 2002 Oxidative effects of LPS on human hemoglobin (Eur. J. Biochem. 269) 4637


























