
Transglutaminase-mediated polyamination of vasoactive intestinal
peptide (VIP) Gln16 residue modulates VIP/PACAP receptor activity
Salvatore De Maria
1
, Salvatore Metafora
2
, Vittoria Metafora
2
, Francesco Morelli
2
,
Patrick Robberecht
3
, Magalı
`Waelbroeck
3
, Paola Stiuso
4
, Alfredo De Rosa
1
, Anna Cozzolino
4
,
Carla Esposito
4
, Angelo Facchiano
5
and Maria Cartenı
`
1
1
Department of Experimental Medicine and Centro di Ricerca Interdipartimentale di Scienze Computazionali e Biotecnologiche, II
University of Naples, Italy;
2
CNR Institute of Genetics and Biophysics Adriano Buzzati Traverso, Naples, Italy;
3
Department of
Biochemistry and Nutrition, Medical School of Medicine, Universite
´Libre de Bruxelles, Bruxelles, Belgium;
4
Department of
Chemistry, University of Salerno, Salerno, Italy;
5
Istituto di Scienze dell¢Alimentazione, CNR, Avellino, Italy
Previous data showing an increase of receptor binding
activity of [R16]VIP, a vasoactive intestinal peptide (VIP)
structural analogue containing arginine at the position 16 of
its amino acid sequence, have pointed out the importance of
a positive charge at this site. Here, the functional charac-
terization of three VIP polyaminated adducts (VIP
Dap
,
VIP
Spd
, and VIP
Spm
), obtained by a transglutaminase-
catalysed reaction between the VIP Gln16 residue and
1,3-diaminopropane (Dap), spermidine (Spd), or spermine
(Spm), is reported. Appropriate binding assays and adeny-
late cyclase enzymatic determinations have shown that these
VIP adducts act as structural VIP agonists, both in vitro and
in vivo. In particular, their IC
50
and EC
50
values of human
and rat VIP/pituitary adenylate cyclase activating peptide
(PACAP)
1
and VIP/PACAP
2
receptors indicate that VIP
Dap
is a VIP agonist, with an affinity and a potency higher than
that of VIP, while VIP
Spd
and VIP
Spm
are also agonists but
with affinities lower than that of VIP. These findings suggest
that the difference in adduct agonist activity reflects the
differences in the positive charge and carbon chain length of
the polyamine covalently linked with the VIP Gln
16
residue.
In addition, the data obtained strongly suggest that the
length of polyamine carbon chain could be critical for the
interaction of the agonist with its receptor, even though
possible hydrophobic interaction cannot be ruled out. In vivo
experiments on murine J774 macrophage cell cultures have
shown the ability of these compounds to stimulate the
inducible nitric oxide synthase activity at the transcriptional
level.
Keywords: NO/iNOS; polyamines; transglutaminase; VIP
agonists; VIP receptors.
Vasoactive intestinal polypeptide (VIP) is a 28-amino acid
long peptide that serves the function of hormone, neuro-
transmitter, and immuno-modulator in mammals and other
vertebrates. It belongs to the important family of brain/gut
hormones including secretin, glucagon, pituitary adenylate
cyclase activating peptide (PACAP), etc. [1–3]. Although
originally identified on the basis of its strong vasodilating
activity, VIP exerts a wide spectrum of biological effects on
a number of target organs mediated by its interaction with
two distinct G-protein coupled receptors (VIP/PACAP
1
and VIP/PACAP
2
or VPAC
1
and VPAC
2
), which transduce
the ligand signal through the activation of different
enzymatic effector systems, such as adenylate cyclase,
phospholipase C, and inducible nitric oxide synthase (iNOS)
[4–9].
While work is more advanced on the mechanism of
ligand binding and activation of G-protein coupled recep-
tors which use relatively small molecules as their ligands,
fewer results are available in the case of peptide receptors
which have ligands that are much larger and which exhibit
greater conformational flexibility. The detailed mechanism
of signal transduction mediated by the VIP receptor and the
physiological role of the different VIP receptors are
currently investigated. Furthermore, the only structural
information available on VIP has been mainly obtained by
CD and NMR analysis [10]. Recently, a conformational
study explored the theoretically preferred conformation of
VIP by combining experimental information with unre-
strained molecular calculation. The results of these studies
showed that (a): most VIP conformations, including the
global minimum, can be described as bent conformation; (b)
atype1bturn involves the residues of the VIP fragment
P2–5 and a different type of b-turn involves the residues of
the fragment P6–11; (c) the central portion (residues 7–15)
and the C-terminus (residues 19–27) are in ahelical confor-
mation [11,12].
Little is known on the role played by the different VIP
residues in the recognition and activation of natural
receptors. Structural–activity studies, performed on a
Correspondence to S. Metafora, CNR International Institute of
Genetics and Biophysics, Via Pietro Castellino, 111-80131 Naples,
Italy. Fax: + 39 081 6132 253, Tel.: + 39 081 6132 254,
E-mail: metafora@iigbna.iigb.na.cnr.it
Abbreviations:MEM,minimalessentialmedium;CHO,Chinese
hamster ovary; Dap, 1,3-diaminopropane; iNOS, inducible nitric
oxide synthase; LPS, lipopolysaccharide; L-NAME, N
x
-nitro-
L
-arginine methyl ester; NO, nitric oxide; PACAP, pituitary adenylate
cyclase activating peptide; Pt, putrescine; Spd, spermidine; Spm,
spermine; TGase, transglutaminase; VIP, vasoactive intestinal pep-
tide; VPAC
1
, VIP/PACAP
1
receptor; VPAC
2
, VIP/PACAP
2
receptor.
(Received 11 February 2002, revised 14 May 2002,
accepted 15 May 2002)
Eur. J. Biochem. 269, 3211–3219 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.02996.x

number of analogues and different VIP fragments, demon-
strated that full action of VIP is critically dependent on the
integrity of the entire molecule [13]. The VIP N-terminal
helix is known to be critical for the high affinity binding and
coupling to the effector system, while the C-terminal
sequence has been shown to be important for VPAC
1
and
VPAC
2
discrimination [14–17]. Concerning the central
region of the VIP polypeptide chain, different amino acid
substitutions at this site did not affect the VIP affinity or
potency, suggesting that this region is not directly involved
in the recognition or activation of receptors. In contrast,
Robberecht et al. demonstrated the unexpected importance
of Gln16 in the central region of the secretin family peptides
for its interaction with the receptor N-terminal domain [18].
On the basis of this finding, we were prompted to use the
transglutaminase (TGase) to modify the primary structure
of VIP in order to investigate the effect of insertion at the
level of the Gln16 c-carboxyamide group of a variety of
amines of different carbon chain length and positive charge
on VPAC receptor activity, both in rats and humans [19–
23]. The functional characterization of three polyaminated
VIP derivatives demonstrated their ability to act as agonists
with an affinity and a potency higher than VIP (VIP
Dap
)or
with an affinity lower than VIP (VIP
Spd
and VIP
Spm
)on
VPAC receptors. The relevance of the polyamine carbon
chain length and positive charge on receptor activation has
been pointed out and the results of some experiments on
murine J774 macrophage cell cultures have shown the
ability of these VIP adducts to modulate in vivo the iNOS
activity at the level of transcription.
MATERIALS AND METHODS
VIP and Ro 25-1553 synthesis
These peptides were synthesized as C-terminal amides by
solid phase methodology on an automated Applied
Biosystem apparatus using Fmoc chemistry as described
previously [24]. The peptides were cleaved and purified by
RP-HPLC on an apparatus using a DBV 300A (10 ·1cm)
column and by ion exchange chromatography on a Mono S
HR 5/5 column. Peptide purity (95%) was assessed by
capillary electrophoresis and sequence conformity was
verified by direct sequencing and ion spray MS.
TGase-catalysed synthesis of VIP derivatives
TGase activity was preliminarily assayed by determining the
Ca
2+
-dependent covalent binding of amines to the VIP
peptide acting as amino acceptor substrate. Analysis of the
reaction products was performed by SDS/PAGE, followed
by fluorography [25,26], using radioactive putrescine (Pt),
spermidine (Spd) or spermine (Spm) as amino donor
substrates.
Each preparation of c-(glutamyl16)-Dap-VIP (VIP
Dap
),
c-(glutamyl16)-Spd-VIP (VIP
Spd
), and c-(glutamyl16)-Spm-
VIP (VIP
Spm
) was obtained by incubating for 12 h in a final
volume of 200 lLat37C50lgofnativeVIPwithTGase
in 125 m
M
Tris/HCl buffer, pH 8.0, containing 10 m
M
dithiothreitol, 2.5 m
M
CaCl
2
, and 0.2
M
Dap or Pt, or
Spd, or Spm, where required; 3 lg (6.7 mU) TGase were
added at the start of incubation, and the same amount of
enzyme was added after 6 h. A control sample incubated in
the absence of TGase was assayed simultaneously. At the
end of the incubation, the reaction mixtures were centrifuged
at 12 000 gfor 10 min, and the resulting supernatants were
used to purify the VIP analogues by HPLC.
Purification and characterization of the VIP derivatives
The VIP analogues present in the supernatants were purified
by HPLC chromatography (Waters; Model 660 HPLC
apparatus) using an analytical reversed-phase Vydac C18
column (4.6 ·150 mm; Separations Group, Hesperia, CA).
The column was equilibrated with 0.01% trifluoroacetic
acid and elution was performed in 35 min (flow rate
1mLÆmin
)1
) at room temperature with a 0–60% aceto-
nitrile linear gradient. Fractions of 0.2 mL were collected
and the absorbance peaks were pooled and evaporated to
dryness. The dry samples were dissolved in distilled water
and submitted to ES-MS, as described previously [27].
CHO cell line culture
The recombinant Chinese hamster ovary (CHO) cells
expressing the rat or human recombinant VPAC
1
and
VPAC
2
receptors were prepared in P. Robberecht’s labor-
atory (Department of Biochemistry and Nutrition, Medical
School of Medicine, Universite
´Libre de Bruxelles, Bel-
gium). Cells were incubated at 37 Cina-minimal essential
medium (a-MEM), supplemented with 10% fetal bovine
serum, 2 m
ML
-glutamine, 100 lgÆmL
)1
penicillin and
100 lgÆmL
)1
streptomycin, with an atmosphere of 95%
air and 5% CO
2
. Geneticin (0.4 mgÆmL
)1
) was always
present in the culture medium of stock cultures. Subcultures
used for membrane purification were grown in a medium
without geneticin.
Membrane preparation, receptor identification,
and adenylate cyclase determination
An appropriate number of CHO cells was harvested with
a cell scraper and pelleted by low speed centrifugation, the
supernatant was discarded and the sedimented cells were
lysed by addition of 1 m
M
NaHCO
3
and quick freezing in
liquid nitrogen. After thawing, the lysate was centrifuged
at 4 C for 10 min at 400 gand the supernatant was
further centrifuged at 20 000 gatthesametemperature
andforthesametimelength.Thefinalpelletwas
resuspended in 1 m
M
NaHCO
3
and used immediately as a
crude membrane preparation. [
125
I]VIP (specific radio-
activity, 0.7 mCiÆmmol
)1
) was used as tracer for the
identification of both rat or human VPAC
1
receptors;
[
125
I]Ro 25–1553 (specific radioactivity, 0.8 mCiÆmmol
)1
)
was used as tracer for labelling the rat or human VPAC2
receptors [28]. The binding of labelled ligands to purified
CHO membranes was performed as described [14]; in all
cases the nonspecific binding was defined as the residual
binding in the presence of 1 l
M
VIP. Competition curves
were carried out by incubating membranes and tracer in
the presence of increasing concentrations of unlabelled
peptides. Peptide potency was expressed as IC
50
value, i.e.
as the peptide concentration required for half maximal
inhibition of tracer binding. In detail, the binding was
performed at 37 C in a buffer containing 20 m
M
Tris/
maleate pH 7.4, 2 m
M
MgCl
2
, 0.1 mgÆmL
)1
bacitracin,
3212 S. De Maria et al. (Eur. J. Biochem. 269)FEBS 2002

and 1% BSA; 3–30 lg protein was used per assay. The
bound radioactivity was separated from the free radioac-
tivity by filtration through glass fibre filters GF/C
presoaked for 24 h in 0.1% polyethyleneimine and rinsed
three times with a 20 m
M
phosphate buffer (pH 7.4)
containing 1% BSA. Adenylate cyclase activity was
determined by a previously published technique [29].
Membrane proteins (3–15 lg)wereincubatedinatotal
volume of 60 lL containing 0.5 m
M
[a-
32
P]ATP, 10 l
M
GTP, 5 m
M
MgCl
2
, 0.5 m
M
EGTA, 1 m
M
cAMP, 1 m
M
theophylline, 10 m
M
phosphoenolpyruvate, 30 lgÆmL
)1
pyruvate kinase, and 30 m
M
Tris/HCl at a final pH of
7.5. The reaction was initiated by membrane addition and
was terminated after a 12-min incubation at 37 Cby
adding 0.5 mL of stop buffer (0.5% SDS, 0.5 m
M
ATP,
0.5 m
M
cAMP, 20 000 c.p.m. [8
3
H] cAMP). cAMP was
separated from ATP by two successive chromatographies
on Dowex 50-WX8 and neutral alumina.
Macrophage cell culture
The murine monocyte/macrophage cell line J774 (ATCC
TIB 67) was grown as monolayers in tissue-culture flasks
(75 cm
2
growth area; Falcon) in Dulbecco’s MEM sup-
plemented with 10% (v/v) fetal bovine serum (Euroclone,
UK), 4 m
ML
-glutamine, 100 unitsÆmL
)1
penicillin, and
100 lgÆmL
)1
streptomycin (standard culture medium). Cells
were harvested by gentle scraping and passaged every 3–6
days. For use, cells were seeded into 12-well plates (Falcon)
and allowed to adhere for 2 h. After this, medium was
replaced with fresh medium containing either 0.01 lgÆmL
)1
lipopolysaccharide (LPS; this complex molecule is a com-
ponent of the Gram-negative bacteria outer membrane
possessing a strong iNOS-inducing activity on murine
macrophages) alone (control), or VIP and its polyaminated
adducts (10
)10
)10
)6
M
), alone or in combination with LPS,
and the cells were incubated at 37 C for a further 24 h in an
humidified atmosphere containing 5% CO
2
and 95% air.
Cell viability was measured by both Trypan blue exclusion
test and MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-
nyltetrazolium bromide; Sigma Aldrich]. In specific control
inhibition experiments dexamethasone (10
)6
M
;Sigma)was
added to macrophages treated with either 0.01 lgÆmL
)1
LPS alone, or VIP and its polyaminated adducts
(10
)10
)10
)6
M
), alone or in combination with LPS.
Nitric oxide measurement
The NO produced by the iNOS-catalysed reaction was
evaluated by measuring with the Griess reagent nitrite
released by the macrophages into the culture medium [30].
Following 24 h incubation at 37 C, 400-lL aliquots of
culture medium were taken from the plates containing the
cell monolayers, mixed with an equal volume of Griess
reagent (0.5% sulfanilamide and 0.05% N¢-1-naphtylethy-
lenediamine dihydrochloride in 2.5% phosphoric acid), and
incubated at room temperature for 10 min The absorbance
of the coloured solution was measured at 570 nm. The
amount of nitrites released into culture medium was
expressed as nmol nitrites per 5 ·10
6
cells per 24 h, using
a sodium nitrite curve as a standard. Control experiments
demonstrated that VIP and VIP adducts did not interfere
with the Griess reaction.
Evaluation of iNOS activity
iNOS activity was determined in crude homogenates of
J774 cells. An appropriate number of cells was incubated
for 24 h in the absence or presence of either LPS
(0.01 lgÆmL
)1
) or VIP or its polyaminated adducts
(10
)10
)10
)6
M
), alone or in combination with LPS. After
the end of the incubation time, the cells were rinsed three
times with ice-cold NaCl/Pi, removed from the culture
plates with a cell scraper, collected, and transferred to
microcentrifuge tubes. The sedimented cells were lysed by
addition of 50 lL ice-cold hypotonic homogenization
buffer (1 m
M
EDTA, 1 m
M
hypotonic EGTA, 25 m
M
Tris/HCl pH 7.4). The iNOS activity occurring in 20 lg
of homogenate proteins was evaluated by a NOS Detection
Assay Kit (Stratagene) [31] according to the manufacturer’s
instructions. In this assay, [
3
H]arginine (50 CiÆmmol
)1
;
Amersham) was used as substrate and the reaction mixture
was incubated for 30 min at 37 C. Two blanks were
included in the assay: one was prepared by omitting the
homogenate, the other by adding the iNOS inhibitor
N
x
-nitro-
L
-arginine methyl ester (
L
-NAME; 1 m
M
)tothe
reaction mixture before the homogenate. The iNOS
activity was expressed as citrulline pmolÆmg
protein
)1
Æmin
)1
. Control experiments demonstrated that
VIP and polyamines (Dap, Spd, Spm) did not interfere
with the iNOS activity.
RT-PCR
Messenger RNA, isolated by the mRNA Capture Kit
(Roche Diagnostics) from the J774 macrophages incuba-
ted in the standard culture medium for 24 h in the
presence of either 0.01 lgÆmL
)1
LPS, or VIP and its
polyaminated adducts (10
)10
)10
)6
M
), alone or in combi-
nation with LPS, was transcribed by reverse transcriptase
(Superscript II; Life Technologies) at 37 Cfor1.5h
according to the manufacturer’s protocol (final volume
20 lL). The cDNA contained in 2 lL of this reaction
mixture was amplified in another reaction mixture con-
taining, in a final volume of 25 lL, 10 m
M
Tris/HCl
pH 8.3, 1.5 m
M
MgCl
2
, 50 m
M
KCl, 100 ng of both sense
and antisense primers for iNOS (sense, 5¢-GTTTCT
TGTGGCAGCAGC-3¢;antisense,5¢-CCTCGTGGCT
TTGGGCTCCT-3¢), 100 l
M
deoxynucleoside triphos-
phate, and 1 U Taq DNA polymerase (Roche Diagnos-
tics). The reaction was carried out in a DNA thermal
cycler (Promega). The PCRs were performed with 35
cycles in the exponential phase of amplification and always
started with a 3-min denaturation step at 95 Cand
terminated with a final 7 min step at 72 C. The cycle for
iNOS was 95 C, 45 s; 56 C, 45 s; 72 C, 45 s. The PCR
products were analysed by electrophoresis on a 1.2%
agarose gel in Tris/borate/EDTA [32]. The identities of the
amplification products were confirmed by comparison of
their sizes with the sizes expected from the known gene
sequence. Coamplification of a different cDNA sequence
was performed by adding into the amplification reaction
mixture the b-actin gene primers (10 ng of both sense and
antisense primers: sense, 5¢-CGTGGGCCGCCCTAGG
CACCA-3¢;antisense,5¢-TTGGCCTTAGGGTTCA
GGGGGG-3¢). No products were detectable in control
amplifications performed in the absence of cDNA (data
FEBS 2002 VIP polyaminated agonists and receptor activity (Eur. J. Biochem. 269) 3213
not shown). The semiquantitative evaluation of the PCR
products was achieved by integrating the peak area
obtained by densitometry of the ethidium bromide stained
agarose gels [software used: NIH image V.16; iNOS
(600 bp): 109, 757, 3300, 4581, 1159, 901; b-actin (300 bp):
670, 797, 833, 963, 819, 831]. The ratio between the yield
of each amplified product and coamplified b-actin (iNOS/
b-actin mRNA ratio: 0.162, 0.949, 3.961, 4.757, 1.415,
1.084) allows a relative estimate of mRNA levels in the
samples analysed.
Multiple alignment and charge distribution in receptor
sequences
The amino acid sequences of the VIP receptors analysed
were derived from the SwissProt database. The following
sequences were used for the multiple alignment analysis:
VIPR_CARAU (VPAC
1
goldfish), VIPR_HUMAN
(VPAC
1
human), VIPR_PIG (VPAC
1
pig), VIPR_RAT
(VPAC
1
rat), VIPS_HUMAN (VPAC
2
human), VIPS_
MOUSE (VPAC
2
mouse), VIPS_RAT (VPAC
2
rat). The
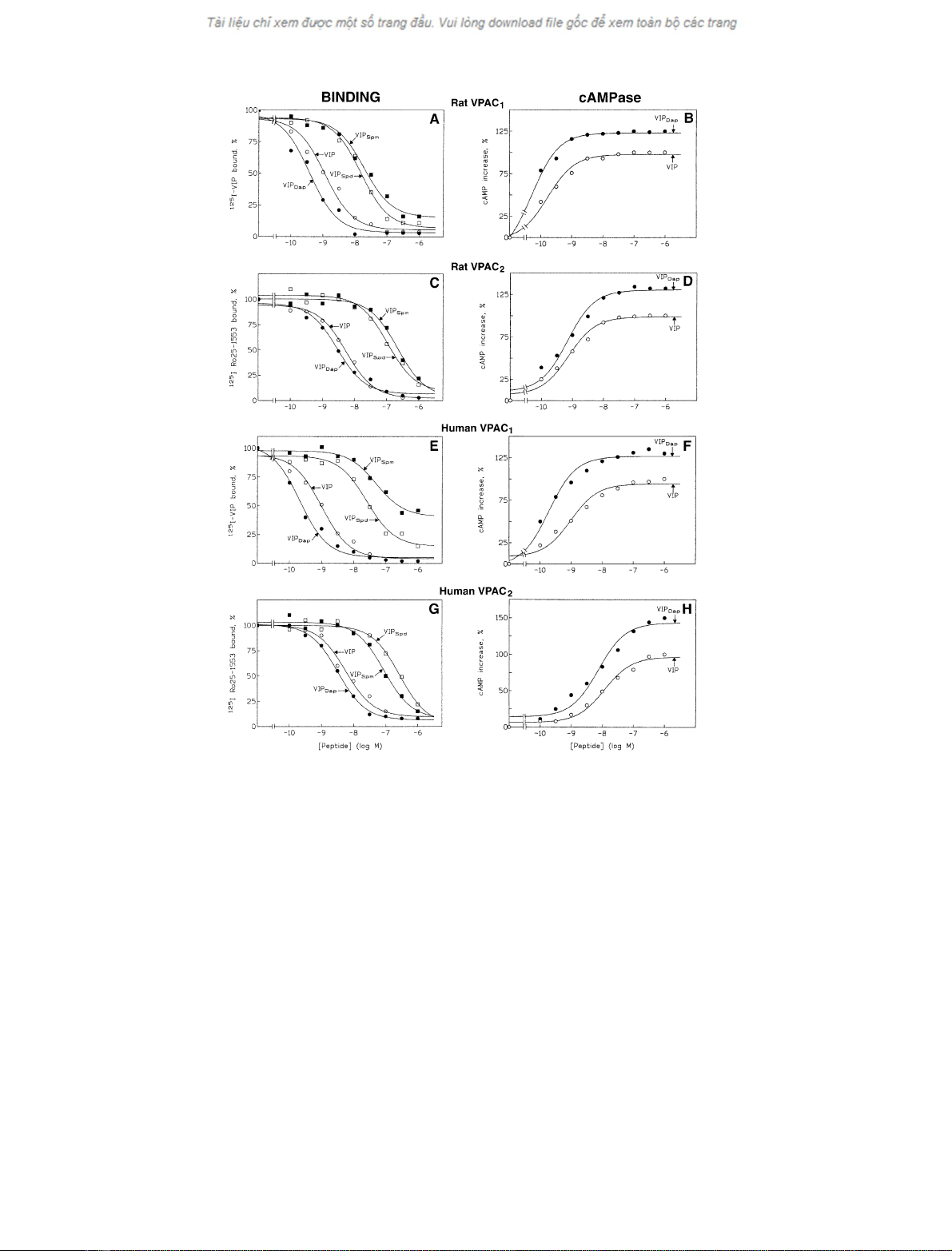
Fig.1. EffectofVPAC
1
and VPAC
2
ligands (VIP and its polyaminated agonists) on membrane binding and adenylate cyclase activity. The data
reported in the figure refer to: (1) Dose-dependent inhibition of
125
I-labelled ligand ([
125
I]VIP was used for the identification of rat or human VPAC
1
receptors, whereas [
125
I]Ro 25-1553 was used for labelling of rat or human VPAC
2
receptors) binding (panels A, C, E, and G) to crude preparations
of CHO cell membranes expressing recombinant VPAC
1
and VPAC
2
receptors, by VIP (s), VIP
Dap
(d),VIP
Spd
(h), and VIP
Spm
(j); the results
are the means of three different determinations and are expressed as the percentage of tracer specifically bound; (2) Dose-effect curves of VIP (s),
VIP
Dap
(d) on adenylate cyclase activation (B, D, F, and H) in crude preparations of membranes from CHO cells expressing recombinant VPAC
1
and VPAC
2
receptors; the results, expressed in percentage increase of
32
P-labelled cyclic AMP produced in the presence of 1 l
M
VIP, are the means
of three different experiments. The cAMPase activity was evaluated by a previously published radiometric assay [29]. Further experimental details
are reported in Materials and methods.
3214 S. De Maria et al. (Eur. J. Biochem. 269)FEBS 2002
multiple alignment was created by the
CLUSTALW
software.
Colours were added manually by considering the common
colour-code for charged amino acids (i.e. red for acidic, and
cyan/blue for basic side chains). The analysis of charge
distribution in the extracellular and cytoplasmatic domains
was carried out by considering the domain assignment
reported in the SwissProt database.
Statistical analysis
The data have been reported as means ± SEM of at least
three different determinations. The means were compared
using analysis of variance (one-way
ANOVA
)plusBonfer-
roni’s t-test and a P-value < 0.05 was considered significant.
The software packages used for statistical analysis were
GRAPHPAD INSTAT
and
MINITAB
. The curve fitting programs
used were in
GRAPHPAD PRISM
,
GRAPHPAD INPLOT
, and
MINITAB
.
RESULTS
A positively charged amino acid into position 16
modulates the VIP ability to bind its specific receptors
VIP and its three polyaminated adducts (VIP
Dap
, VIP
Spd
,
and VIP
Spm
) possessing a different positively charged side
chain at position 16, were first characterized by appropriate
binding experiments to VPAC receptors, both in humans
and rats. The data reported in Fig. 1 (panels A, C, E, G)
and analysed in Table 1 demonstrate that the VIP
Dap
adduct has a higher affinity (lower IC
50
value) than VIP on
both rat and human VPAC
1
receptors, and a similar
affinity to VIP on VPAC
2
receptors. The VIP
Spd
and
VIP
Spm
derivatives were 30–100-fold less potent than VIP.
The effect of the agonists used in these experiments was
tested on VPAC
1
and VPAC
2
receptors in both rat and
human on the assumption that the analysis of the data
obtained, associated with the knowledge of the structural
differences between these two receptors and between the rat
and human VPAC
1
receptors [33], could allow a better
identification of the polypeptide regions involved in the
ligand/receptor molecular interactions.
The polyaminated VIP adducts are agonists of either
higher or lower affinity and potency than VIP
The effect of the three polyaminated VIP adducts on the
human and rat VPAC
1
and VIPAC
2
receptor activity was
evaluated by evaluating the adenylate cyclase enzymatic
activity of a crude preparation of membranes. The data
reported in Fig. 1 (panels B, D, F, H) and Table 1 indicate
that VIP
Dap
has a higher apparent affinity and higher
maximum effect than VIP in all the receptors tested. In
contrast, VIP
Spd
and VIP
Spm
werefoundtoactwithalower
apparent affinity, their EC
50
values being 3–10 times higher
than the VIP value (Table 1). The data on the relative
potencies of Spd- and Spm-conjugated VIP in cAMP
generation assays (not shown in Fig. 1) indicate that the
decrease in biological activity of these adducts reflects the
apparent decrease in their binding affinity at the lowest
concentrations used (10
)10
)10
)7
M
), even though at the
highest concentrations (10
)7
)10
)6
M
) the biological activity
improves significantly. By comparing the IC
50
and EC
50
of
Table 1. IC
50
and EC
50
values (n
M
) from binding experiments and adenylate cyclase assays. Experimental details are described in Materials and methods. Values are means ± SEM and are the means of at least
three different determinations. IC
50
, Peptide concentration (n
M
) required for 50% tracer binding inhibition; EC
50
, peptide concentration (n
M
) required for half maximal stimulation of adenylate cyclase activity;
IA, intrinsic activity, the ratio between the maximal stimulating effect of modified VIP and that of VIP. *P<0.05,**P< 0.01 (Bonferroni’s t-test) vs. the VIP value.
Human Receptor Rat Receptor
VPAC
1
VPAC
2
VPAC
1
VPAC
2
Ligand IC
50
EC
50
IA IC
50
EC
50
IA IC
50
EC
50
IA IC
50
EC
50
IA
VIP 1.0 ± 0.2 2 ± 0.2 1.0 10 ± 3.0 7.0 ± 1.0 1.0 1.1 ± 0.1 0.6 ± 0.1 1.0 5 ± 1.0 1.0 ± 1.0 1.0
VIP
Dap
0.3 ± 0.1* 0.8 ± 0.1* 1.3 ± 0.10 5 ± 0.8** 7.0 ± 0.8 1.5 ± 0.2 0.4 ± 0.1** 0.2 ± 0.1* 1.2 ± 0.2 3 ± 0.5* 0.6 ± 1.0 1.3 ± 0.2
VIP
Spd
40 ± 3** 15 ± 4** 1.1 ± 0.15 200 ± 18** 120 ± 10** 1.0 ± 0.1 15 ± 2** 2 ± 0.5** 1.1 ± 0.1 150 ± 20** 20 ± 3** 1.1 ± 0.1
VIP
Spm
200 ± 50** 90 ± 50** 0.9 ± 0.15 100 ± 15** 80 ± 12** 0.9 ± 0.1 20 ± 4** 4 ± 2** 1.0 ± 0.2 260 ± 15** 60 ± 8** 1.0 ± 0.1
FEBS 2002 VIP polyaminated agonists and receptor activity (Eur. J. Biochem. 269) 3215



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





