
Evidence for general stabilization of mRNAs in response to UV light
Frank Bollig*, Reinhard Winzen*, Michael Kracht, Beniam Ghebremedhin, Birgit Ritter, Arno Wilhelm,
Klaus Resch and Helmut Holtmann
Institute of Pharmacology, Medical School Hannover, Germany
mRNA stabilization plays an important role in the changes
in protein expression initiated by inducers of inflammation
or direct cell stress such as UV light. This study provides
evidence that stabilization in response to UV light differs
from that induced by proinflammatory stimuli such as
bacterial lipopolysaccharide or interleukin (IL)-1. Firstly,
UV-induced stabilization is independent of the p38 MAP
kinase pathway, which has previously been shown to medi-
ate stabilization induced by IL-1 or lipopolysaccharide.
UV-induced mRNA stabilization was insensitive to the
dominant negative forms of p38 MAP kinase and its sub-
strate MAP kinase-activated protein kinase 2 (MK2), or to
the p38 MAP kinase inhibitor SB 203580, demonstrating
that it occurs through a different signaling mechanism.
Secondly, UV-induced stabilization exhibits a different
transcript selectivity. Activation of the p38 MAP kinase
pathway, by expressing active MAP kinase kinase 6, induced
stabilization only of transcripts containing AU-rich
elements. UV light also induced stabilization of transcripts
lacking AU-rich elements. This effect could not be mimicked
by expressing MEKK1, an upstream activator of the p38,
JNK, ERK and NF-jB pathways. UV light also stabilized
endogenous histone mRNA, which lacks AU-rich elements
and a poly(A) tail. This effect was not mimicked by active
MAP kinase kinase 6 and not sensitive to a p38 MAP kinase
inhibitor. This suggests that UV light induces stabilization
through a mechanism that is independent of p38 MAP
kinase and affects a broad spectrum of mRNAs.
Keywords: AU-rich element; MAPKAP kinase 2; mRNA
stability; p38 MAP kinase; UV light.
Higher organisms respond to an external insult by switching
on the expression of certain genes the products of which are
involved in the defense against pathogens and in tissue
repair. Pathogen-derived material, direct cell stress and
endogenous mediators activate gene expression at multiple
levels, including transcriptional activation as well as post-
transcriptional mechanisms. The importance of the latter
has been demonstrated in gene-targeted mice where over-
production of inflammatory proteins due to dysregulation
of mRNA degradation or translation caused severe disease
of the animals [1–5].
The molecular basis underlying the regulation of mRNA
translation and decay is not completely understood. An
important type of regulatory mRNA element are AU-rich
elements (AREs), which are found in the 3¢-UTRs of many
rapidly inducible genes such as oncogenes and cytokine genes
[6,7]. By imposing rapid degradation on the transcript, the
AREs limit basal expression and allow rapid reversion to
basal mRNA levels subsequent to gene induction. Stability
and translation of ARE-containing transcripts can be
affected by signaling mechanisms activated by the damaging
agents directly or by released inflammatory cytokines.
Cell stressors, infectious pathogens and inflammatory
cytokines activate various signaling pathways simulta-
neously. Extensive overlap exists in the sets of pathways
activated by the different agents. Pathways activated include
NF-jB and the mitogen-activated protein (MAP) kinase
cascades. The JNK pathway has been reported to stabilize
the short-lived interleukin (IL)-2 mRNA on activation of the
T-cell line Jurkat [8] and the IL-3 mRNA in the murine mast
cell line PB-3c [9]. Several groups have shown an mRNA-
stabilizing effect of protein kinase C activation and/or
increased intracellular Ca
2+
concentrations [6,8–12]. The
results of others, including our own, show that stabilization
of several ARE-containing mRNAs, triggered by IL-1 or
bacterial lipopolysaccharide (LPS), involves activation of
p38 MAP kinase [13–17] and its substrate MAP kinase-
activated protein kinase 2 (MK2) [16–18]. Consistent with
these findings, MK2-deficient mice exhibit reduced synthesis
of several cytokines in response to LPS [19].
Similarly to LPS and IL-1, UV light is a potent inducer of
inflammation and induces expression of numerous genes
including cytokines and oncogenes [20,21], which is in part
due to the stabilization of mRNAs [22,23]. UV light
strongly activates stress signaling pathways, including the
p38/MK2 pathway [24]. However, the signaling mecha-
nisms involved in mRNA stabilization in response to UV
light have not been identified, nor has the transcript
selectivity of UV-induced stabilization been defined.
In this study, we show that, in HeLa cells, mRNA
stabilization induced through the p38/MK2 pathway is
Correspondence to H. Holtmann, Institute of Pharmacology,
Medical School Hannover, Carl-Neuberg Strasse-1,
D-30625 Hannover, Germany.
Fax: + 49 511 5324081, Tel.: + 49 511 5322800,
E-mail: holtmann.helmut@mh-hannover.de
Abbreviations: ARE, AU-rich element; GFP, green fluorescent pro-
tein; GM-CSF, granulocyte–macrophage colony-stimulating factor;
IL, interleukin; LPS, lipopolysaccharide; MAP, mitogen-activated
protein; MK2, MAP kinase-activated protein kinase 2 (also named
MAPKAP kinase 2); MKK6, MAP kinase kinase 6.
*Note: these two authors contributed equally to this work.
(Received 5 July 2002, revised 2 October 2002,
accepted 8 October 2002)
Eur. J. Biochem. 269, 5830–5839 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03300.x

limited to AU-rich transcripts, whereas UV light stabilizes a
broad spectrum of mRNAs either containing or lacking
AU-rich elements by a mechanism that is independent of the
p38/MK2 pathway.
MATERIALS AND METHODS
Cells and materials
HeLa cells constitutively expressing the tet transactivator
protein [25] (kindly provided by H. Bujard, Center for
Molecular Biology, University of Heidelberg, Germany)
were cultured in Dulbecco’s modified Eagle’s medium
complemented with 5% fetal bovine serum. Plasmids ptet-
BBB-I18
972)1310
and ptet-BBB-GM-CSF
ARE
encode the
rabbit b-globin mRNA with AU-rich regions of the Il-8
and GM-CSF transcripts respectively inserted into the
BglII site of the b-globin 3¢-UTR [16,26]. The pUHC13-3
plasmid, kindly donated by H. Bujard, contains the
Photinus pyralis luciferase cDNA downstream of a
tetracycline-regulated promoter [25]. pUHD10-CAT-
TIMP1 was generated by excising the IL-8 fragment of
pUHD10-CAT-IL-8 [16] with BamHI and inserting a
fragment of human TIMP1 (nucleotide 19–782, accession
no. NM_003254) generated by RT-PCR with primers
containing BamH1 sites. To obtain pUHD10-GFP a
fragment of pEGFP-C1 (Clontech) including the green
fluorescent protein (GFP) cDNA and 3¢adjacent restric-
tion sites was amplified with XbaI-flanked primers and
inserted into the XbaI site of pUHD10.3 [25]. Expression
plasmids for constitutively active MAP kinase kinase 6
(MKK6
2E
), dominant negative p38, dominant negative
and constitutively active MK2 have been described [16].
To generate HeLa cells with inducible expression of
active MKK6, the MKK6
2E
cDNA was placed in-frame
downstream of the GFP cDNA in pUHD10-GFP. HeLa
cells were cotransfected with this plasmid and a plasmid
for puromycin resistance, and stable transfectants selected
by culture in 1 lgÆmL
)1
puromycin. Myc-tagged HuR
was expressed with the plasmid pTet-Myc-over-HuR [27]
(a gift from A.-B. Shyu, University of Texas, Houston,
TX, USA). Rabbit antiserum against AUF1 was a gift
from G. Brewer, University of Medicine and Dentistry of
New Jersey, Piscataway, NJ, USA. Mouse monoclonal
antibodies 19F12 against HuR and 9H10 against hnRNP
A1 were kindly donated by H. Furneaux, University of
Conneticut Health Center, Farmington, CT, USA, and
G. Dreyfuss, University of Pennsylvania School of
Medicine, Philadelphia, PA, USA, respectively.
Transfections and reporter assays for mRNA stability
Transient transfections by the calcium phosphate method
and RNA degradation kinetics were performed as
described [16]. Briefly, cells (5 ·10
6
seeded per 9-cm-
diameter dish) were transfected with the indicated plas-
mids. Amounts of plasmid DNA within each experiment
were kept constant by adding empty vector. For each
kinetics of mRNA degradation assay, cells from one dish
were trypsinized and distributed into parallel cultures to
ensure equal transfection efficiency within the group of
samples. The next day transcription from the tetracycline-
regulatable promoter was stopped by addition of
doxycycline (3 lgÆmL
)1
). At the indicated times thereaf-
ter, total RNA was isolated, and Northern-blot analysis
was performed using digoxigenin-labeled antisense RNA
probes. RNA half lives were determined as in [16], using
a video imaging system and the
MOLECULAR ANALYST
program (Bio-Rad).
Preparation of cytoplasmic extracts
Cytoplasmic extracts were prepared as described by Wang
et al. [22]. All steps were carried out in the cold. The cells
(10
6
per sample) were washed once with NaCl/P
i
,harvested
by scraping, pelleted by centrifugation, and resuspended in
200 lL hypotonic buffer (10 m
M
Hepes, pH 7.9, 10 m
M
KCl, 1.5 m
M
MgCl
2
,1lgÆmL
)1
leupeptin, 1 lgÆmL
)1
aprotinin and 0.5 m
M
phenylmethanesulfonyl fluoride).
Then 25 lL of the same buffer including 2.5% (v/v)
Nonidet P-40 was added. After centrifugation at 1000 g
for 4 min, the supernatants were removed, freeze–thawed
five times, and cleared by centrifugation. Aliquots were
frozen at )70 C.
In vitro
transcription and electrophoretic mobility-shift
assays
Labeled RNA (typically 10
7
)10
8
c.p.m.Ælg
)1
) was synthes-
ized by incubating 1–3 lg linearized plasmid DNA in a
mixture of 50 lCi [a-
32
P]UTP (400 CiÆmmol
)1
, Hartmann
Analytic, Braunschweig, Germany), 18 l
M
unlabeled UTP,
0.5 m
M
unlabeled ATP, CTP and GTP, 2 UÆlL
)1
T7 RNA
polymerase or T3 RNA polymerase as required (both
enzymes from Roche) and 2 UÆlL
)1
RNase inhibitor (MBI)
for 1 h at 37 C. RNase-free DNase (Roche) was then
added to a final concentration of 1 UÆlL
)1
. After incuba-
tion for 15 min at 37 C the RNA was passed through a
NucTrap push column (Stratagene) to remove free nucle-
otides, and stored at )80 C. Radiolabeled RNA probes
(1.5 ·10
5
c.p.m.) were incubated with cytoplasmic extracts
(6 lg protein per sample) in 20 lL buffer containing 20 m
M
Hepes, pH 7.9, 100 m
M
KCl, 2 m
M
MgCl
2
, 3% (v/v)
glycerol, 0.5 m
M
dithiothreitol, 0.5 m
M
phenyl-
methanesulfonyl fluoride, 5 lgÆmL
)1
pepstatin A and
200 lgÆmL
)1
tRNA for 10 min at 30 C. RNase T1 (30
units/sample) was then added, and incubation continued for
20 min at 37 C. Where indicated, antibodies were included
for the last 10 min. Samples were electrophoresed on a
nondenaturing polyacrylamide gel (5% acrylamide in
0.25 ·Tris/borate/EDTA buffer). The gels were dried and
autoradiographed.
Western blot and
in vitro
kinase assay
HeLa cells were lysed in 20 m
M
Hepes, pH 7.5, containing
50 m
M
KCl, 2 m
M
MgCl
2
,0.5m
M
dithiothreitol, 0.5 m
M
phenylmethanesulfonyl fluoride, 5 lgÆmL
)1
pepstatin,
5lgÆmL
)1
leupeptin, 30 m
M
NaF, 15 m
M
b-glycerophos-
phate and 0.2% Nonidet P-40. After 10 min on ice, lysates
were centrifuged for 5 min at 10 000 g, and supernatants
were saved (cytoplasm). Expression of GFP-MKK6
2E
was
analyzed by Western blotting as described elsewhere [16].
Briefly, cytoplasmic proteins were separated by SDS/PAGE
and electrophoretically transferred to poly(vinylidene
difluoride) membranes (Immobilon-PTM;Milipore).After
FEBS 2002 General mRNA stabilization by UV light (Eur. J. Biochem. 269) 5831
blocking with 5% dried milk in Tris-buffered saline, the
membranes were incubated with monoclonal antibodies
against GFP (Roche Diagnostics) for 16 h, washed and
incubated with peroxidase-coupled second antibody.
GFP-MKK6
2E
was detected by using the SuperSignal
chemiluminescence system (Pierce). For in vitro kinase
assays, 20 lg cytoplasmic proteins were diluted in kinase
buffer (20 m
M
Tris/HCl, pH 7.4, 5 m
M
MgCl
2
,0.2m
M
dithiothreitol, 0.1 m
M
EDTA, 0.1 m
M
EGTA, 20 m
M
b-glycerophosphate, 1 m
M
phenylmethanesulfonyl fluoride,
10 l
M
ATP), and 4 lCi [c-
32
P]ATP and 1 lg recombinant
HSP27 (kindly provided by Matthias Gaestel, Medical
School Hannover, Germany) were added. After 30 min at
30 C, SDS/PAGE sample buffer was added, and samples
were boiled for 5 min and separated by SDS/PAGE.
Phosphorylated HSP27 was detected by autoradiography
of the dried gel.
RESULTS
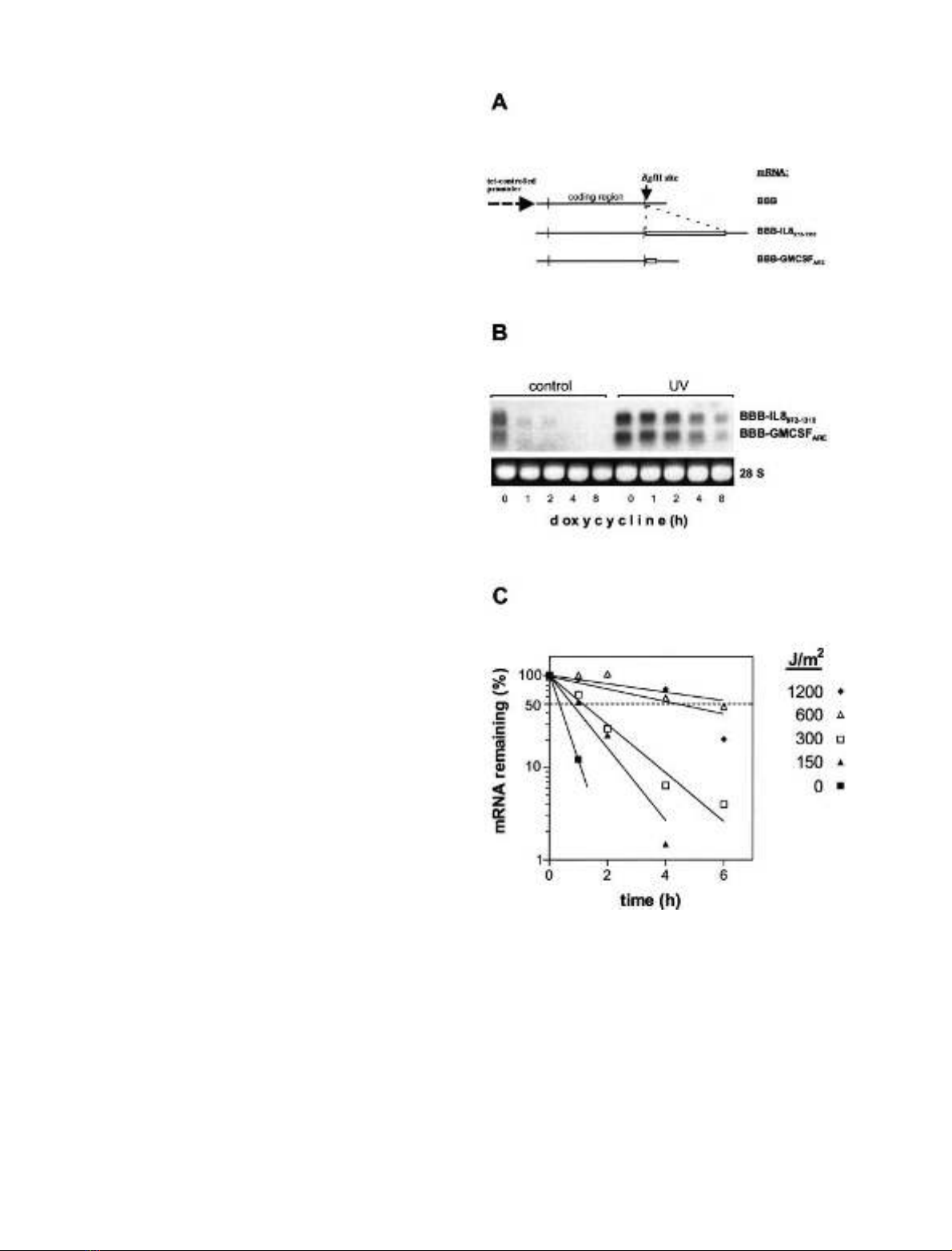
UV light induces stabilization of AU-rich mRNAs
independently of the p38 MAP kinase/MK2 pathway
Mechanisms that affect mRNA turnover were studied in
HeLa-tTA cells expressing the tetracycline-sensitive trans-
activator [25]. The decay of mRNAs expressed with the
tet-off system was followed subsequent to inhibition of their
transcription by the tetracycline analog doxycycline. The
b-globin mRNA exhibits a long half-life under these
conditions (> 10 h [16], and additional data, not shown).
Decay of ARE-containing transcripts was investigated by
expressing b-globin reporter constructs containing the
regulatory region of the IL-8 3¢-UTR (BBB-IL-8
972)1310
)
and, to minimize the chance of detecting effects specific only
for that region, with the well characterized ARE of
GM-CSF (BBB-GMCSF
ARE
). In agreement with previous
studies [6,16,26], the mRNAs derived from both constructs
were rapidly degraded in unstimulated cells (Fig. 1B).
Exposure to UV light (UV-B) induced marked and dose-
dependent stabilization of both hybrid mRNAs (Fig. 1B,C).
According to kinetic studies, the increase in stability
persisted for about 14 h after exposure to UV light and
gradually disappeared thereafter (not shown).
As reported previously [16], activators of the p38
MAP kinase/MK2 pathway induce stabilization of
AU-rich mRNAs, including BBB-IL-8
972)1310
and BBB-
GMCSF
ARE
(Fig. 1A). To determine whether mRNA
stabilization induced by UV light also involved the p38
MAP kinase pathway, dominant-negative mutants of p38
MAP kinase (p38
AGF
) or MK2 (MK2
K76R
)werecoex-
pressed (Fig. 2A). As expected, the expression of each of
the mutants strongly interfered with the stabilization
induced by MKK6
2E
, a selective activator of p38 MAP
kinase. In contrast, neither the dominant-negative p38
MAP kinase mutant nor the dominant negative MK2
mutant affected stabilization induced by UV light
(Fig. 2A). In support of this result, the pyridinyl imidazole
SB 203580, a selective p38 MAP kinase inhibitor, inhibited
mRNA stabilization induced by MKK6
2E
, whereas it did
not have a significant effect on stabilization by UV light
(Fig. 2B). The data indicate that stabilization in response to
UV light occurs independently of the p38 MAP kinase/
MK2 pathway.
Fig. 1. UV light induces stabilization of AU-rich mRNAs. (A) Scheme
of mRNAs expressed. b-Globin mRNAs with the ARE of GM-CSF
(BBB-GMCSF
ARE
) (Fig. 1A) or with an ARE-containing region of
IL-8 mRNA (BBB-IL-8
972)1310
) were expressed under the control of a
tetracycline-regulatable promoter (for details see Materials and
methods). (B) HeLa cells constitutively expressing the tet transactiva-
tor protein were transfected with ptetBBB-IL-8
972)1310
and ptetBBB-
GM-CSF
ARE
. At 2 h after UV-B exposure (1200 JÆm
)2
), doxycycline
(3 lgÆmL
)1
) was added. Total RNA was isolated at the indicated times
and analyzed by Northern blotting. Ethidium bromide staining of 28S
rRNA is shown to allow comparison of RNA amounts loaded. (C)
Quantification of results for BBB-IL-8
972)1310
mRNA from an
experiment performed as in (B), but with different doses of UV light.
5832 F. Bollig et al.(Eur. J. Biochem. 269)FEBS 2002
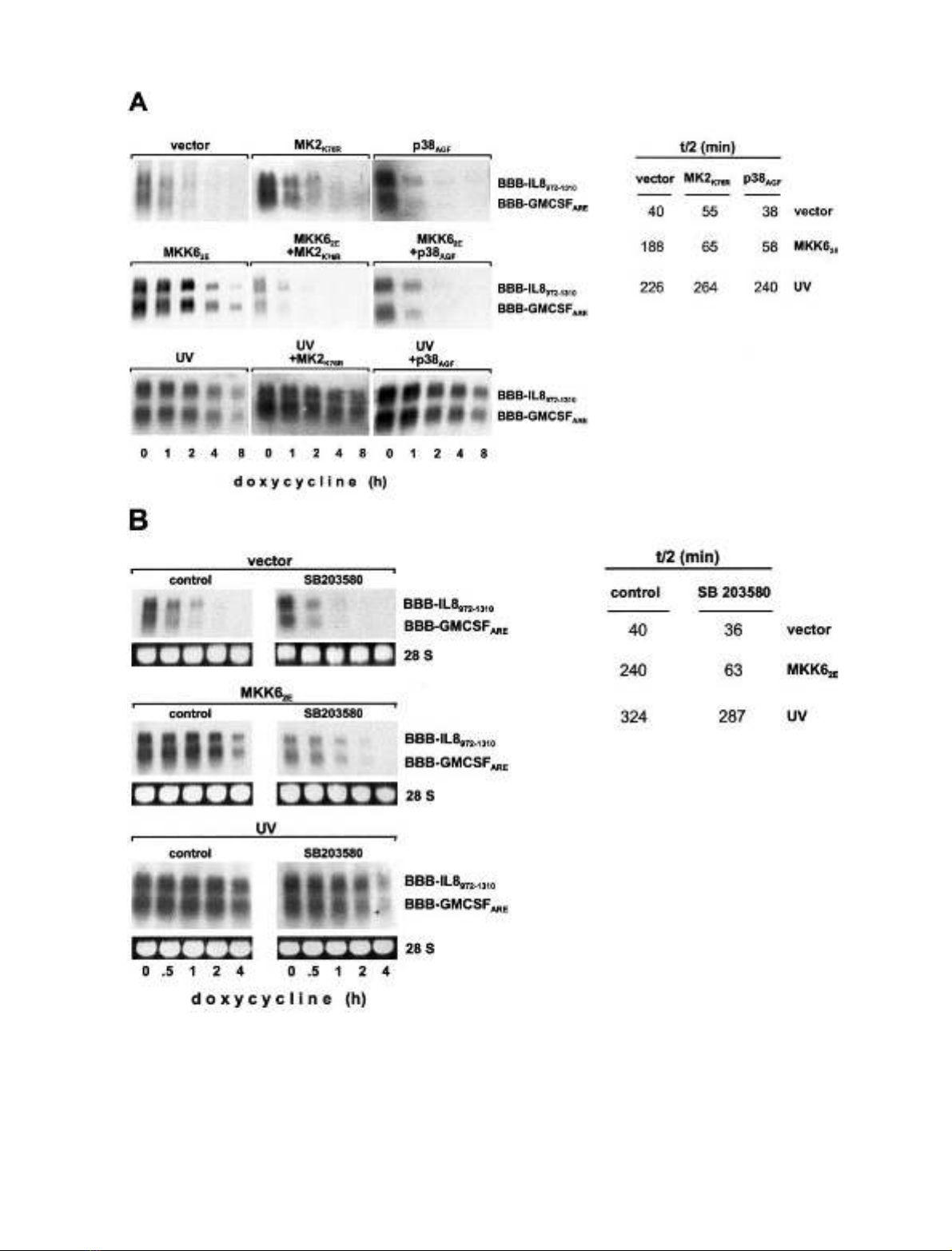
Fig. 2. mRNA stabilization by UV light is independent of the p38/MK2 pathway. Degradation of BBB-IL-8
972)1310
and BBB-GM-CSF
ARE
tran-
scripts was determined as in Fig. 1 in untreated or UV-exposed HeLa cells cotransfected with empty vector or expression vectors for constitutively
active MKK6 (MKK6
2E
). (A) Plasmids encoding dominant negative p38 MAP kinase (p38
AGF
)ordominantnegativeMK2(MK2
K76R
)were
cotransfected as indicated. (B) Cells received SB 203580 (2 l
M
) or vehicle 3 h before the assay of RNA stability. Half-lives for BBB-IL-8
972)1310
were quantified as described in Materials and Methods.
FEBS 2002 General mRNA stabilization by UV light (Eur. J. Biochem. 269) 5833
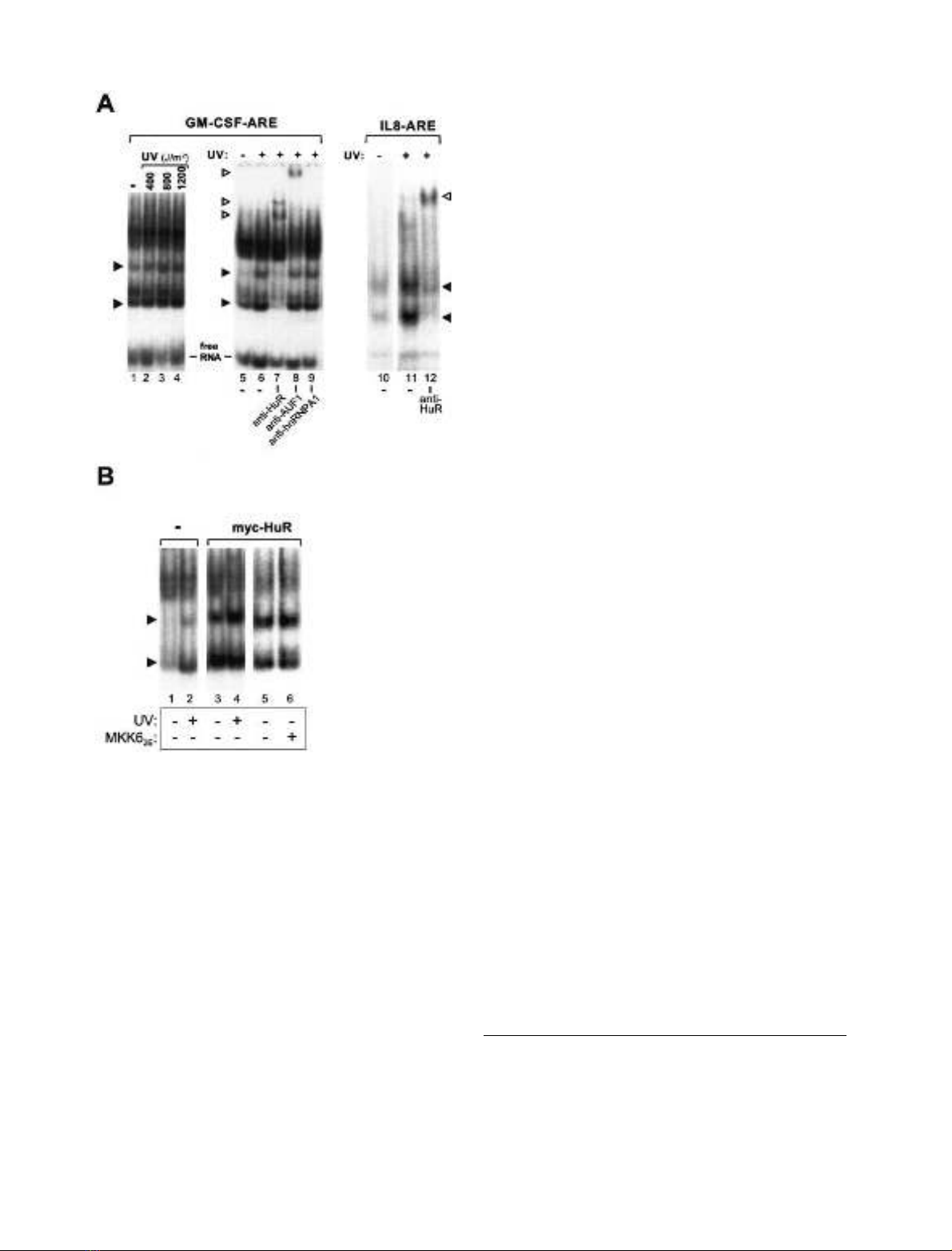
UV light but not activation of p38 MAP kinase increases
cytoplasmic HuR-binding activity
Analysis of protein–RNA binding showed that several
complexes were formed when GM-CSF ARE is incubated
with cytoplasmic extracts from control cells (Fig. 3A, lane
1). Formation of two of the complexes increased with
cytoplasmic extracts from UV-treated cells (Fig. 3A, lanes
1–4). It has been reported recently that UV light induces
translocation of the RNA-binding protein HuR to the
cytoplasm, an effect that contributes to UV-induced stabi-
lization of p21/WAF message [22]. In accordance with that
study, the two complexes modulated by UV light were
supershifted by antibodies against HuR (Fig. 3A, lanes
5–7). Antibodies against AUF1 induced a supershift of a
different complex which was not influenced by UV treat-
ment (lane 8), whereas antibodies against hnRNP-A1 did
not affect any of the complexes (lane 9). The increase in
formation of HuR-containing complexes on exposure to
UV light was also observed with the AU-rich IL-8 mRNA
fragment (Fig. 3A, lanes 10–12). Expression of Myc-tagged
HuR increased the amounts of both UV-inducible com-
plexes, and a further increase was observed in response to
UV light (Fig. 3B, compare lanes 1 and 2 with lanes 3 and
4). On the other hand, active MKK6 did not result in any
detectable change in the amounts of complexes containing
coexpressed Myc-tagged HuR (Fig. 3B, lanes 5 and 6), nor
in the pattern of other complexes formed with the RNA
(additional results, not shown).
UV light but not p38 MAP kinase activation induces
stabilization of non-AU-rich mRNAs
The observation that stabilization of mRNAs on exposure
to UV light is independent of the p38 MAP kinase pathway
suggested that the mechanism of stabilization itself may be
different. To elucidate this, transcript selectivity of the two
ways of inducing stabilization was compared. For this
purpose, additional mRNAs lacking AU-rich sequences
were included in the experiments (Fig. 4). The GFP mRNA
contains a short 3¢-UTR that consists of vector-derived
sequences. The luciferase mRNA derived from the plasmid
pUHC13-3 [25] harbors a long 3¢-UTR with regions of high
A + U content, but with no overlapping AUUUA motifs
nor UUAUUUA U/A U/A motif suggested to confer
regulation of stability [28–30]. The TIMP1 cDNA was
cloned downstream of a 196-nucleotide CAT fragment to
express a CAT-TIMP1 hybrid RNA that can be distin-
guished from endogenous TIMP1 transcript. The 3¢-UTR
of TIMP1 is short (96 nucleotides) and devoid of AU-rich
regions. The basal half-life of the GFP transcript is long
(5 h), whereas that of luciferase and CAT-TIMP1 tran-
scripts is rather short (Fig. 4A). Expression of MKK6
2E
induced stabilization of the two ARE-containing mRNAs
(BBB-GMCSF
ARE
and BBB-IL8
972-1310
) but failed to exert
any effect on the degradation rate of the GFP, luciferase
and CAT-TIMP1 mRNAs (Fig. 4A). In contrast, exposure
to UV light resulted in stabilization of all mRNAs
investigated, including the short-lived CAT-TIMP1 and
luciferasemRNAsaswellasthelong-livedGFPmRNA,
Fig. 3. Effects of UV light and activation of p38/MK2 on complex
formation between AU-rich mRNAs and cytoplasmic proteins. (A) HeLa
cells were left untreated or exposed to UV light (doses 1200 JÆm
)2
or as
indicated). Cytoplasmic extracts were prepared and incubated with
radiolabeled in vitro-transcribed RNAs consisting of the ARE of
GM-CSF or the AU-rich region of the IL-8 3¢-UTR (nucleotides
972–1310). Where indicated, antibodies specific for HuR (0.75 lg),
AUF1 (1 lL of a 1 : 10 dilution of serum) or hnRNP A1 (1 lLofa
1 : 10 dilution of ascites) were included. Protein–RNA complexes were
separated on nondenaturing polyacrylamide gels and detected by
autoradiography. Filled arrowheads indicate UV-induced complexes,
and open arrowheads indicate complexes supershifted by antibodies.
(B) HeLa cells transfected with expression vectors for Myc-tagged
HuR or MKK6
2E
as indicated were left untreated or exposed to UV
light (1200 JÆm
)2
). Interaction of proteins from cytoplasmic extracts
with labeled GM-CSF ARE RNA was assayed as described in (A).
Fig. 4. UV light and the p38/MK2 pathway induce mRNA stabilization
with different transcript selectivities. (A) Degradation of the indicated
mRNAs, all expressed under the control of a tetracycline-regulatable
promoter, was determined in HeLa cells expressing MKK6
2E
or
exposed to UV light as described in Fig. 1. (B) Degradation of the
indicatedmRNAswascomparedincellstransfectedwithanexpres-
sion vector for MEKK1Dor with empty vector as control.
5834 F. Bollig et al.(Eur. J. Biochem. 269)FEBS 2002


























