
AACE CLINICAL CASE REPORTS Vol 5 No. 6 November/December 2019 e339
Copyright © 2019 AACE
Case Report
PROINSULIN-PREDOMINANT PANCREATIC
NEUROENDOCRINE TUMOR-INDUCED HYPOGLYCEMIA
AFTER ROUX-EN-Y GASTRIC BYPASS SURGERY
Khary Edwards, MD; Lori de La Portilla, DO; Faryal S. Mirza, MD; Pooja Luthra, MD
Submitted for publication March 28, 2019
Accepted for publication July 2, 2019
From the Division of Endocrinology and Metabolism, University of
Connecticut School of Medicine, Farmington, Connecticut.
Address correspondence to Dr. Pooja Luthra, Division of Endocrinology and
Metabolism, University of Connecticut School of Medicine, 263 Farmington
Avenue, Farmington, CT 06030.
E-mail: luthra@uchc.edu.
DOI:10.4158/ACCR-2019-0148
To purchase reprints of this article, please visit: www.aace.com/reprints.
Copyright © 2019 AACE.
ABSTRACT
Objective: To present a case of recurrent hypoglyce-
mia following Roux-en-Y gastric bypass (RYGB) surgery
whose etiology was determined to be a proinsulin-predom-
inant pancreatic neuroendocrine tumor (a proinsulinoma).
Methods: A case report along with a brief discussion
and review of the pertinent literature is presented.
Results: The patient is a 62-year-old female who
presented with symptomatic hypoglycemia 11 years after
RYGB surgery. Initial workup revealed low insulin levels
with elevated proinsulin levels. A 72-hour fast confirmed
the presence of proinsulin-induced hypoglycemia second-
ary to a pancreatic neuroendocrine tumor (PNET). She
underwent distal pancreatectomy with splenectomy and a
PNET tumor was successfully removed with resolution of
her symptoms.
Conclusion: Hypoglycemia after RYGB surgery is a
well-established syndrome. While there are several etiolo-
gies for this, PNETs (including proinsulinomas) should be
considered in the differential diagnosis in this population.
Proinsulinomas are an increasingly recognized cause of
hypoglycemia. Proinsulin levels must always be included
as part of the workup of hypoglycemia in an adult. (AACE
Clinical Case Rep. 2019;5:e339-e343)
Abbreviations:
MEN1 = multiple endocrine neoplasia type 1; MRI =
magnetic resonance imaging; NIPHS = noninsulinoma
pancreatogenous hypoglycemia syndrome; PNET =
pancreatic neuroendocrine tumor; RYGB = Roux-en-Y-
gastric bypass
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) are islet
cell-derived neoplasms of the pancreas that produce
hormonally active peptides including insulin, gastrin,
glucagon, and vasoactive intestinal peptide (1). They
represent the most common neuroendocrine tumor (2).
Insulinomas, neuroendocrine tumors which produce insu-
lin, represent the most common type of functional PNETs
and are the most common cause of hypoglycemia associat-
ed with a tumor (3). Proinsulin is produced by the pancre-
atic beta cells and converted into equimolar amounts of C
peptide and insulin via enzymatic cleavage. The biological
activity of proinsulin is 10% that of insulin (4).
Recent advances in laboratory assays make the differ-
entiation between proinsulin and insulin possible (5). This
has led to the discovery that proinsulin-producing PNETs
(proinsulinomas) that predominantly secrete proinsulin
with normal or near-normal levels of insulin secretion can
also cause hypoglycemia (5,6). In 2017, only 16 cases of
proinsulin-secreting PNETs had been reported (6). While
there have been cases of insulinomas in patients after
Roux-en-Y gastric bypass (RYGB) surgery (7), there are
no reports of proinsulinomas after RYGB surgery. We pres-
ent a case of hypoglycemia secondary to a proinsulinoma
after RYGB surgery.
e340 Proinsulinoma after RYGB, AACE Clinical Case Rep. 2019;5(No. 6) Copyright © 2019 AACE
CASE REPORT
A 62-year-old female developed symptoms of hypo-
glycemia (sweating, palpitations, shaking, and nausea)
with episodes of witnessed loss of consciousness several
months prior to presentation. Symptoms were prominent
early in the morning, in fasting conditions, or at night and
resolved after eating. She snacked frequently to avoid
symptoms. She did not give a definite history of weight
change related to her symptoms. She also had a history of a
stable, 1.2-cm non-secreting pituitary macroadenoma and
RYGB surgery 11 years prior to presentation with a total
weight loss of 45 kg. There was no family history of hyper-
calcemia, hypoglycemia, or endocrine tumors. She denied
use of insulin or oral hypoglycemic agents.
She had 1 episode of documented hypoglycemia at her
primary care physician’s office, with loss of consciousness
and a finger stick blood glucose of 28 mg/dL. She respond-
ed quickly to glucose treatment. Fasting laboratory evalu-
ation performed on a separate day showed normal serum
glucose (99 mg/dL), creatinine, liver function, C peptide,
and insulin levels. Proinsulin level was elevated (43.2
pmol/L; reference range is ≤8.0 pmol/L). Serum sulfonyl-
urea screen was negative.
Cosyntropin stimulation testing was performed to
assess for possible adrenal insufficiency. Baseline serum
cortisol level was 11 µg/dL. One hour after intramuscu-
lar injection of 250 µg of cosyntropin, serum cortisol level
appropriately increased to 23 µg/dL, excluding adrenal
insufficiency. In addition, serum total and ionized calcium,
25-hydroxyvitamin D, and parathyroid hormone levels
were normal.
She was admitted for a supervised 72-hour fast. Her
fasting serum blood glucose was 88 mg/dL, C peptide
was 0.9 ng/mL, insulin was 3 µIU/mL, but proinsulin was
elevated at 26.8 pmol/L (reference range is ≤8.0 pmol/L).
At hour 72, she developed symptomatic hypoglycemia
with venous blood glucose of 43 mg/dL. Laboratory test-
ing revealed that insulin and C peptide levels were appro-
priately low, sulfonylurea screen was negative, but proin-
sulin level was elevated (Table 1). Her beta hydroxybutyr-
ate level was elevated at 1.4 mmol/L (reference range is
0.0 to 0.3 mmol/L). Low plasma insulin concentration with
elevated proinsulin levels indicated a proinsulinoma or a
predominantly proinsulin-secreting pancreatic tumor. She
received glucagon at the end of the fast. Her blood glucose
did not change and remained at 43 mg/dL at 10, 20, and 30
minutes after glucagon injection.
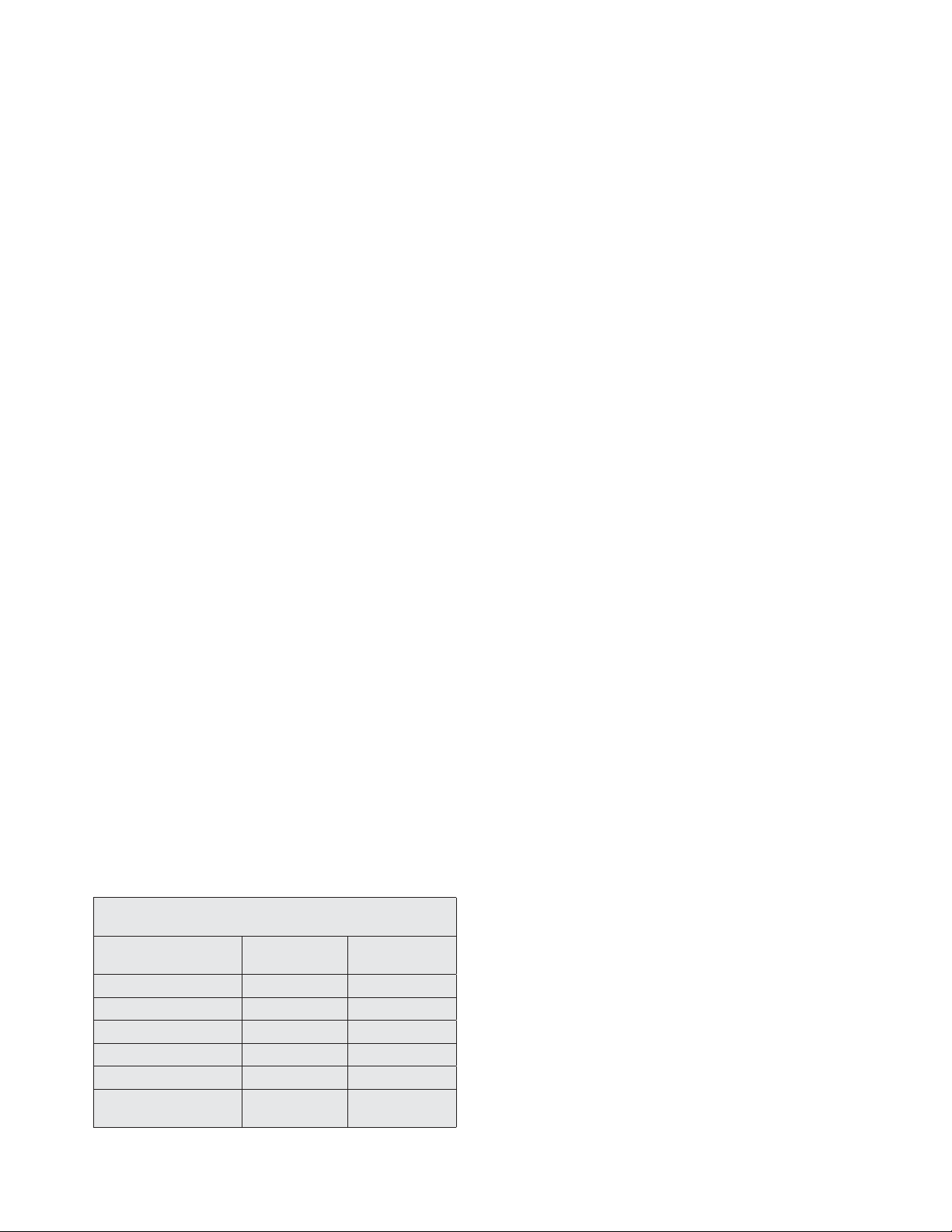
Magnetic resonance imaging (MRI) of the abdomen
(with and without contrast) was undertaken and demon-
strated a 2.4-cm, mildly hypervascular lesion in the pancre-
atic tail and a 0.9-cm, uncinate process lesion with mild
T2 hyperintensity (Fig. 1). An indium (In-111) pentet-
reotide scan performed was negative for a pentetreotide-
avid neoplasm.
She underwent an open distal pancreatectomy with
splenectomy to remove the tumor without complication.
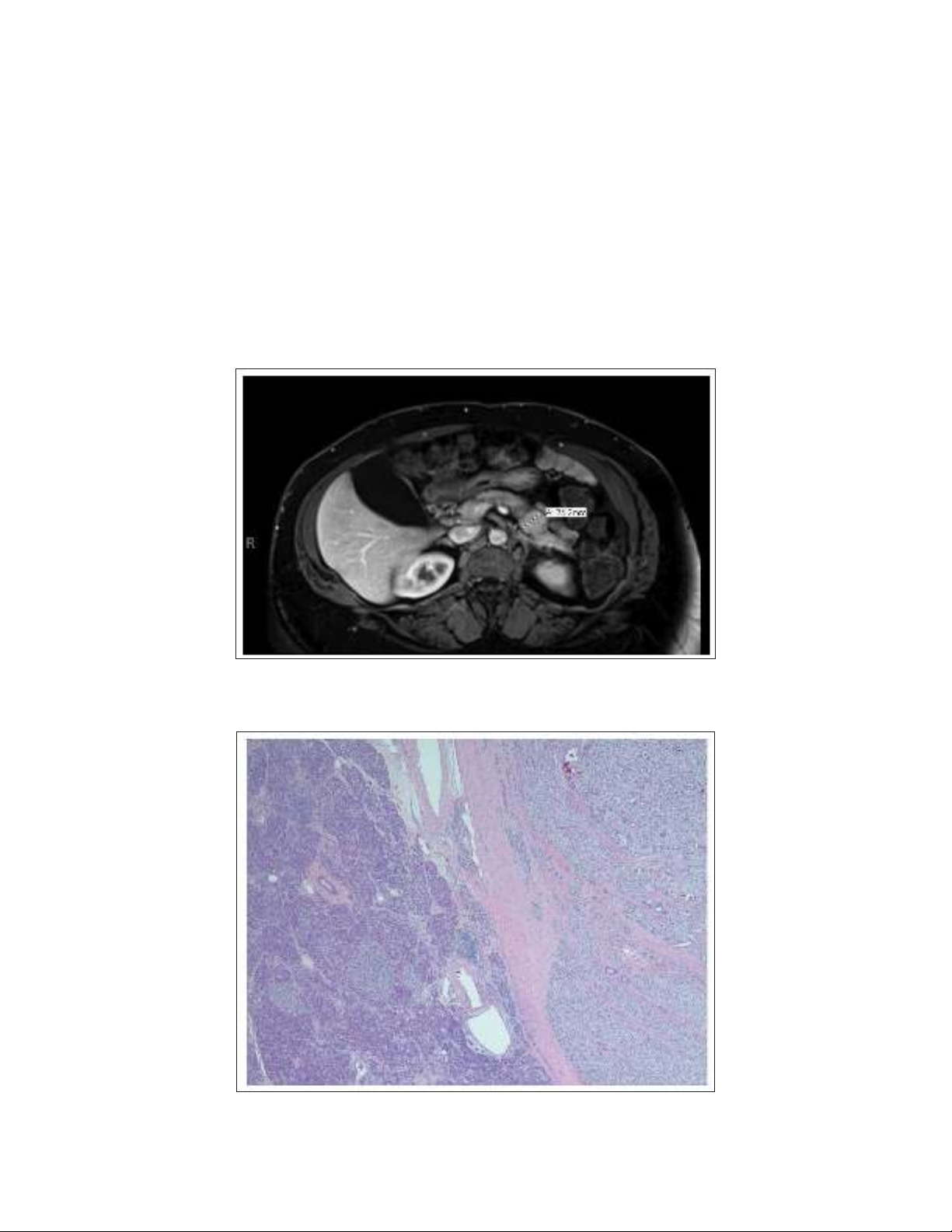
Histopathology revealed a 2.5 × 2.4 × 2.2-cm, unifocal
tumor from the pancreatic body (Fig. 2) that was immuno-
reactive for insulin (Fig. 3). The antibody stain used was
Novocastra liquid mouse monoclonal antibody (product
code NCL-L-INSULIN, Leica Biosystems Inc., Buffalo
Grove, IL). There was no lympho-vascular or perineural
invasion. The tumor was histologic grade 1 with <2 mitosis
per 2 mm2. Pathologic staging was pT2, pNX, pM N/A.
Spleen and accessory spleen samples were negative for
tumor involvement.
Postoperatively, she did not have any further hypogly-
cemic episodes and lost 7 kg of weight, as she was not
snacking constantly. She has been symptom-free for at
least 1 year of follow up. Fasting proinsulin level 1 month
after surgery was 6.2 pmol/L (reference range is ≤8.0
pmol/L). She was also evaluated by neurosurgery for the
nonfunctioning pituitary macroadenoma and was recom-
mended conservative follow up as serial MRI did not show
any change in size of the adenoma.
Laboratory evaluation of pituitary function showed
normal levels of insulin-like growth factor-1, adrenocor-
ticotropic hormone, thyroid-stimulating hormone, free
thyroxine, and alpha subunit. Her follicle-stimulating
hormone and luteinizing hormone levels were in the
appropriate reference ranges for postmenopausal women.
Prolactin was mildly elevated at 23.79 ng/mL (reference
range is 2.74 to 19.64 ng/mL). Genetic testing for multiple
endocrine neoplasia type 1 (MEN1) by Invitae Corporation
(San Francisco, CA) was negative for sequence changes,
exonic deletions, or mutations in the genes CDKN1B
and MEN1.
DISCUSSION
Our patient presented with symptomatic hypogly-
cemia in the setting of a prior history of RYGB surgery.
Hypoglycemia (venous blood glucose <60 mg/dL) has
been reported after RYGB surgery in up to 13% of patients
in 1 study, with 0.7% of patients having severe hypogly-
Table 1
72-Hour Fast Results
Patient’s value
at hour 72
Reference
range
Beta-hydroxybutyrate 1.4 mmol/L 0.0-0.3 mmol/L
C peptide 0.5 ng/mL 0.8-3.9 ng/mL
Glucose 43 mg/dL ≤100 mg/dL
Insulin 2 µIU/mL 2.0-19.6 µIU/mL
Proinsulin 26.4 pmol/L ≤8 pmol/L
Serum sulfonylurea
screen Negative None
Proinsulinoma after RYGB, AACE Clinical Case Rep. 2019;5(No. 6) e341 Copyright © 2019 AACE
Fig. 1. Magnetic resonance image of the abdomen and pelvis with and without contrast. There is
a 2.4-cm, mildly hypervascular lesion in the pancreatic tail.
Fig. 2. Insulinoma with adjacent normal pancreas. This figure shows a well-circumscribed and
encapsulated lesion comprised of monotonous-appearing cells on hematoxylin and eosin stain
(×2).
cemia (venous blood glucose <40 mg/dL) (8). Several
mechanisms may contribute to hypoglycemia after RYGB
surgery which have been reviewed by Salehi et al (9). Of
these, dumping syndrome (postprandial hypoglycemia
due to altered delivery of nutrition to the gut [10]) and
noninsulinoma pancreatogenous hypoglycemia syndrome
(NIPHS, a syndrome of postprandial hyperinsulinemic
hypoglycemia due to beta cell hypertrophy [11]) are
well reported.
While these diagnoses were considered in our patient,
she did not have the classic postprandial hypoglycemia
associated with dumping syndrome and NIPHS, but instead
had fasting hypoglycemia, more consistent with her PNET
diagnosis. The possibility of both syndromes coexisting
was considered, but pathology of the resected pancreas
did not display nesidioblastosis or beta cell hypertrophy to
support NIPHS (11).
In our patient, low plasma insulin level with low serum
glucose and high proinsulin was indicative of a proinsulin-
predominant PNET. Most patients with a negative 72-hour
fast have beta-hydroxybutyrate levels >2.7 mmol/L
because of suppressed insulin levels (12). In our case, the
relatively low level of beta-hydroxybutyrate observed in
the setting of normal insulin levels at the time of hypogly-
cemia is likely due to the elevated proinsulin level and its
insulin-like activity.

e342 Proinsulinoma after RYGB, AACE Clinical Case Rep. 2019;5(No. 6) Copyright © 2019 AACE
The patient did not become hypoglycemic until the
end of the 72-hour fast. This has also been reported in the
literature. In a study of 205 patients with insulinoma, 93%
of patients had a positive fast within 48 hours, and 99%
within 72 hours. Hence the recommendation is to do a stan-
dard 72-hour fast to detect the very small percentage of
patients who have a positive fast between 48 and 72 hours.
It is likely that our patient falls into this group (13).
Hyperinsulinemia usually completely suppresses
glycogenolysis and glycogen stores are preserved in
patients with insulinomas due to persistently elevated insu-
lin levels. Therefore, when glucagon is given at the end of
a prolonged fast, blood glucose levels quickly improve due
to glycogenolysis. This was not observed in our patient,
who did not have an increase in glucose with administra-
tion of glucagon after hypoglycemia, at hour 72 of her
fast. Although this is not the usual response with insuli-
nomas, it is possible that because proinsulin only has 10%
of the activity of insulin, she may have had a low level of
glycogenolysis during the 72-hour fast, causing glycogen
stores to be depleted by the end of her fast. This would also
explain the delayed presentation of hypoglycemia at hour
72 of the fast. However, similar findings were not observed
in other reported cases of proinsulin-secreting neuroendo-
crine tumors (6).
There have also been case reports of patients with
insulinomas whose tumor insulin production was appro-
priately suppressed during prolonged fasting, but have
hyperinsulinemic hypoglycemia when given glucose or
glucagon (14). In these cases, the patients did not develop
neuroglycopenic symptoms during the 72-hour fast, and
had appropriate fasting ketosis and elevated beta-hydroxy-
butyrate production. However, this was not observed in our
patient. Proinsulinomas are rare occurrences, and if not for
advances in laboratory assays that are now able to differ-
entiate between insulin and proinsulin, these tumors would
be identified as insulinomas. In our patient, the tumor was
identified as an insulin-secreting tumor, since the antibody
used for staining has a specificity for human insulin but
cannot identify proinsulin (Fig. 3).
Since proinsulin is the precursor molecule to insulin,
one may expect higher insulin levels in tumors secreting
proinsulin. Proinsulinomas however, appear to secrete
intact proinsulin molecules that are not enzymatically
cleaved, unlike insulin secreted from native pancreatic beta
cells and insulinomas. This was demonstrated in a review
of 16 cases with the majority of the cases having signifi-
cantly elevated fasting proinsulin levels in the setting of
normal C peptide and insulin levels (6).
MEN1 was also a consideration in our patient as she
presented with 2 of the common tumors associated with
MEN1, both a PNET tumor and a pituitary adenoma.
MEN1 can be diagnosed clinically in patients who pres-
ent with 2 of the 3 classic tumors (parathyroid, pituitary,
and pancreatic tumors) (15). However, she denied a fami-
ly history of pancreatic, pituitary, or parathyroid tumors
as well as hypercalcemia. MEN1 genetic testing was
also negative.
For bariatric surgery patients, an insulinoma (or proin-
sulinoma) should be suspected if they present with symp-
toms of neuroglycopenia (confusion, fatigue, weakness,
syncope) or adrenergic symptoms of hypoglycemia (irrita-
bility, perspiration, palpitations) while fasting or during the
night. This is distinct from hyperinsulinemic hypoglyce-
mia associated with the late dumping syndrome or NIPHS
which occurs in the postprandial state, usually within 1
Fig. 3. Immunohistochemical stain for insulin in the lesion and adjacent pancreas showing cyto-
plasmic expression in the lesion cells (A; ×2) with no expression in the adjacent normal pancreas
(B; ×20).
A B

Proinsulinoma after RYGB, AACE Clinical Case Rep. 2019;5(No. 6) e343 Copyright © 2019 AACE
to 3 hours of eating. Patients may experience weight gain
from the need to constantly eat to prevent their symptoms.
Fasting laboratory evaluation may show inappropriately
elevated levels of insulin, C peptide, or proinsulin with low
blood glucose. This should prompt further evaluation for
the cause of hypoglycemia.
Computed tomography or MRI of the abdomen are
regarded as first-line imaging modalities for localizing
PNETs tumors, including insulinomas and proinsulino-
mas. Multi-detector computed tomography has sensitivity
and specificity ranges from 63 to 82% and 83 to 100%,
respectively, with a detection rate as high as 94.4%. MRI
has sensitivity and specificity ranges between 85 to 100%
and 75 to 100%, respectively, with a detection rate of 50 to
94% (16). While the overall sensitivity of indium (In-111)
pentetreotide scanning for PNETs is 70 to 90%, the sensi-
tivity for insulinomas is 50 to 70% (17). Endoscopic ultra-
sound can also be used to determine the precise location
and provide samples for histopathology. The sensitivity
and specificity of endoscopic ultrasound can range from
83 to 93% and 83.7 to 95%, respectively, for detection of
PNETs and has been used to detect proinsulinomas not
identified on noninvasive imaging (18).
If the suspicion for a PNET remains high, additional
invasive techniques including selective arterial calcium
stimulation can be undertaken. This technique has been
shown to have a detection rate of 88 to 100% for insuli-
nomas (19). However, the response to calcium stimula-
tion in proinsulinomas differs from that of insulinomas.
Proinsulinomas do not show a significant increase in
proinsulin level with calcium stimulation in contrast to the
increase in insulin level with insulinomas (19,20). This
modality may not be as useful in identifying proinsulin-
omas. There are currently no reports to suggest that any
imaging modality can differentiate between insulinomas
and proinsulinomas without histopathologic testing.
CONCLUSION
This case highlights the need to consider proinsulin-
predominant PNETs and insulin-producing PNETs in the
differential diagnoses for hypoglycemia in patients with
history of RYGB surgery. Proinsulinomas remain rare
occurrences, likely due to the lack of assays that can differ-
entiate between insulin and proinsulin. With the advent
of specific assays for proinsulin, we suspect that proin-
sulinomas will be reported more frequently as a cause
of hypoglycemia.
ACKNOWLEDGMENT
We thank the Department of Pathology at Hartford
Hospital in Hartford, Connecticut for providing the images
for Figures 2 and 3.
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
1. Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors:
biology, diagnosis, and treatment. Chin J Cancer. 2013;32:
312-324.
2. Yao JC, Hassan M, Phan A, et al. One hundred years after “carci-
noid”: epidemiology of and prognostic factors for neuroendo-
crine tumors in 35,825 cases in the United States. J Clin Oncol.
2008;26:3063-3072.
3. Iglesias P, Díez JJ. Management of endocrine disease: a clini-
cal update on tumor-induced hypoglycemia. Eur J Endocrinol.
2014;170:R147-R157.
4. Kitabchi AE. Proinsulin and C-peptide: a review. Metabolism.
1977;26:547-587.
5. Chia CW, Saudek CD. The diagnosis of fasting hypoglycemia
due to an islet-cell tumor obscured by a highly specific insulin
assay. J Clin Endocrinol Metab. 2003;88:1464-1467.
6. Murtha TD, Lupsa BC, Majumdar S, Jain D, Salem RR. A
systematic review of proinsulin-secreting pancreatic neuroendo-
crine tumors. J Gastrointest Surg. 2017;21:1335-1341.
7. Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric
surgery: diagnostic dilemma and therapeutic approaches. Obes
Surg. 2016;26:874-881.
8. Goldfine AB, Patti ME. How common is hypoglycemia after
gastric bypass? Obesity (Silver Spring). 2016;24:1210-1211.
9. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after
gastric bypass surgery: current concepts and controversies. J Clin
Endocrinol Metab. 2018;103:2815-2826.
10. van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome
after esophageal, gastric or bariatric surgery: pathophysiology,
diagnosis, and management. Obes Rev. 2017;18:68-85.
11. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-
Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with
nesidioblastosis after gastric-bypass surgery. New Engl J Med.
2005;353:249-254.
12. Service FJ, O’Brien PC. Increasing serum betahydroxybutyrate
concentrations during the 72-hour fast: evidence against hyper-
insulinemic hypoglycemia. J Clin Endocrinol Metab. 2005;90:
4555-4558.
13. Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab.
2000;85:3973-3974.
14. Wiesli P, Schmid C, Perren A, Pfammatter T, Spinas GA,
Keller U. Hypoglycemia in response to glucose and glucagon in
insulinoma patients with a negative prolonged fast: functional and
morphological properties. J Endocrinol Invest. 2004;27:832-838.
15. Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guide-
lines for multiple endocrine neoplasia type 1 (MEN1). J Clin
Endocrinol Metab. 2012;97:2990-3011.
16. Kartalis N, Mucelli RM, Sundin A. Recent developments in
imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol.
2015;28:193-202.
17. Tamm EP, Bhosale P, Lee JH, Rohren EM. State-of-the-art
imaging of pancreatic neuroendocrine tumors. Surg Oncol Clin N
Am. 2016;25:375-400.
18. Rodriguez A, Canto MI, Makary MA. Endoscopic localization
and tattooing of a proinsulinoma for minimally invasive resection.
Pancreas. 2011;40:474-477.
19. Won JG, Tseng HS, Yang AH, et al. Intra-arterial calcium stimu-
lation test for detection of insulinomas: detection rate, responses of
pancreatic peptides, and its relationship to differentiation of tumor
cells. Metabolism. 2003;52:1320-1329.
20. Fadini GP, Maran A, Valerio A, et al. Hypoglycemic syndrome
in a patient with proinsulin-only secreting pancreatic adenoma
(proinsulinoma). Case Rep Med. 2011;2011:930904.


























