http://www.iaeme.com/IJMET/index.asp 1210 editor@iaeme.com
International Journal of Mechanical Engineering and Technology (IJMET)
Volume 10, Issue 03, March 2019, pp. 1210–1216, Article ID: IJMET_10_03_123
Available online at http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=10&IType=3
ISSN Print: 0976-6340 and ISSN Online: 0976-6359
© IAEME Publication Scopus Indexed
THE INFLUENCE OF THE CATHODE SHAPE
ON THE PHASE COMPOSITION AND
STRUCTURE DURING OXIDATION
N. F. Kolenchin
Chief Researcher of Technopolis, Doctor of Engineering, Associate Professor, Industrial
University of Tyumen, Volodarskogo, 38, Tyumen, Russia, 652000
ABSTRACT
The process of anodizing aluminium alloys in an ozone-containing electrolyte when
a flat cathode is replaced with a needle shape was studied. The microstructure of the
oxide layer and the density distribution of the phases in the volume of the film in two
positions of the cathode — stationary and during its rotation — were investigated. The
mechanical properties of the surface layer were determined.
Key words: anodizing, needle-shaped cathode, ozonation, pore microgeometry, phase
distribution, surface hardness, activation.
Cite this Article: N. F. Kolenchin, The Influence of the Cathode Shape on the Phase
Composition and Structure During Oxidation, International Journal of Mechanical
Engineering and Technology 10(3), 2019, pp. 1210–1216.
http://www.iaeme.com/IJMET/issues.asp?JType=IJMET&VType=10&IType=3
1. INTRODUCTION
The search for ways to activate the interelectrode gap during oxidation is one of the promising
areas in the technology of hardening the surface layer of aluminium alloys. For most
researchers, the range of factorial variability is determined by the boundaries of the electrolytic
cell and, as a rule, is associated with changes in the chemical composition of the electrolyte and
the conditions of energy excitation. The performance is estimated by the degree of activity of
the main participants of the process - aluminium and oxygen under the conditions of the
necessary and sufficient influence of the working environment.
Non-traditional is the technology of external activation of oxygen and its transfer into the
interelectrode space. This is achieved through the introduction of ozone into the electrolyte [1].
Being the strongest oxidizer, ozone itself or atomic oxygen formed during its decomposition
acts, contributing to the intensification of the process, changing the structure and phase
composition of the oxide. Questions of the effectiveness of oxide formation in the ozonized
electrolyte are the motive for finding ways to activation, including changing the shape of the
cathode.

N. F. Kolenchin
http://www.iaeme.com/IJMET/index.asp 1211 editor@iaeme.com
With traditional anodizing, a flat-shaped cathode is used [2,3]. This ensures a uniform
distribution of the electric field strength in the electrolytic cell. To change the density of
discharges on the surface of the anode, the amperage or voltage of forming increases.
Study of the influence of the cathode shape on the near-anode space is prompted by the
results of processes of the same nature in which a pointed cathode was used. According to the
authors of [4], the scientific and practical aspects of electrochemical processes in process gases
between the needle-shaped cathode and the liquid anode, when the pores of the oxide layer are
filled with steam, confirm the identity of the processes for anodizing aluminium and its alloys.
During plasma-electrolytic anodizing [5], passing a pulsed current of high density, a thin
cathode is placed over the surface of the electrolyte, and the anode is immersed to a depth of 1
mm. At the time of the breakdown, a vapour-gas funnel with oxygen donors is formed. The rate
of oxidation increases and the phase composition of alumina changes.
Studying the effect of electrode geometry on the distribution of electric fields in a discharge
of a high-current low-inductance vacuum spark type [6], when the cathode was the pointed tip
and the anode was the plane, it was found that the field strength is maximum at the anode
surface at a distance of approximately 1/4 of the radius from the centre, Figure 1.
Figure 1 Distribution of the electric field in the "tip-plane" geometry
During electrospark processing [7], to increase the magnitude of the electric field at one of
the electrodes, the diameter of the second electrode is reduced or sharpened to a radius of
curvature of about 1 micron. This significantly increases the emission of electrons due to the
tunnel effect, which allows you to make high-current installations more reliable and compact,
without the presence of hot electrodes in them.
As a result of the use of filament-like inclusions of titanium and zirconium oriented along
the axis of the needle and facing the emitting surface, the authors of [8] achieved stability of
electron emission.
Needle-shaped cathodes are also used in a vacuum-arc evaporator [9] for surface
metallization due to the evaporation of droplets flying from the cathode, which contributes to
improved adhesion and increased corrosion resistance of surface oxides.
The Influence of the Cathode Shape on the Phase Composition and Structure During Oxidation
http://www.iaeme.com/IJMET/index.asp 1212 editor@iaeme.com
2. MATERIALS AND METHODS
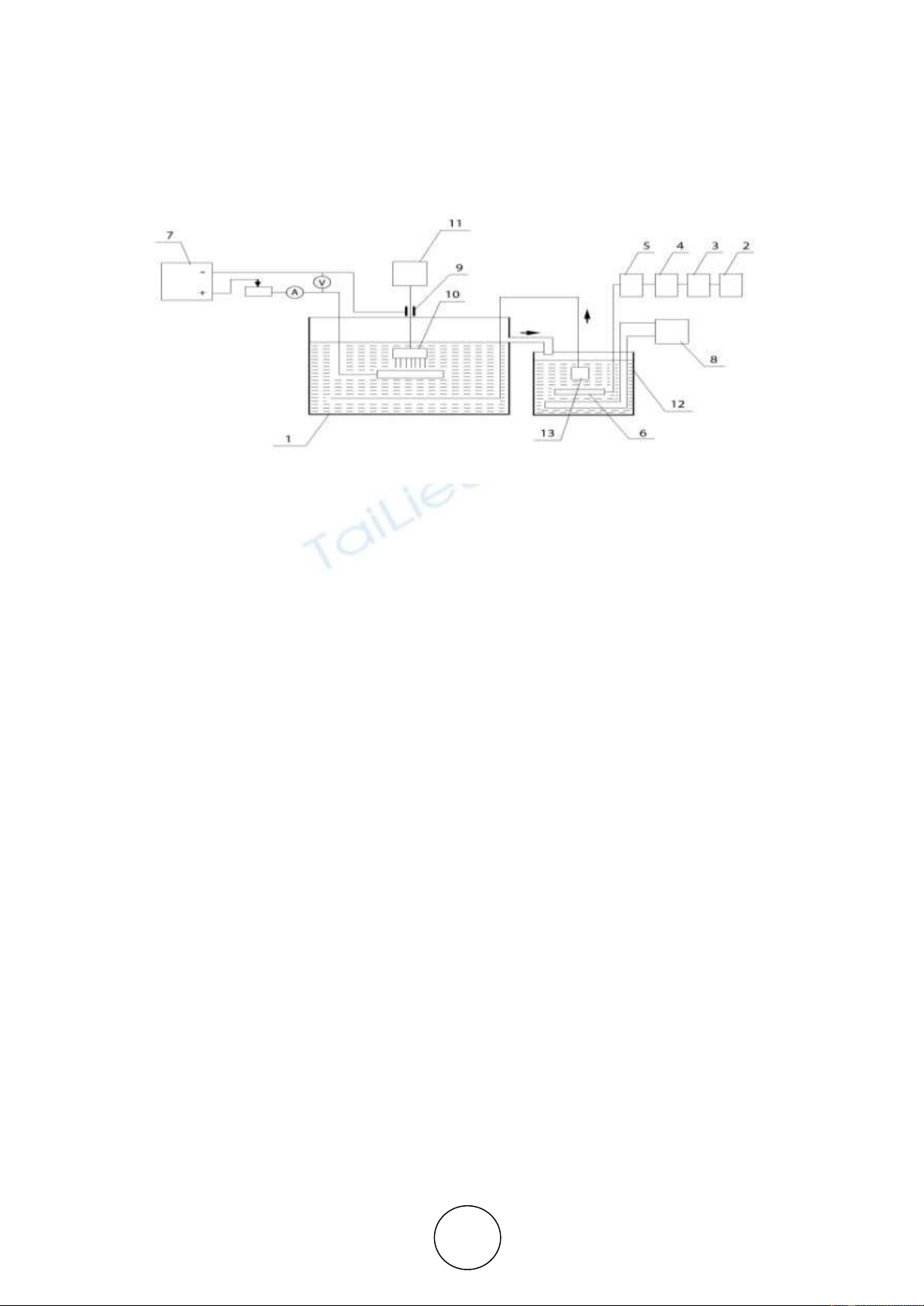
The anodizing scheme is shown in Figure 2.
The anodizing process was carried out in a bath tank (1), made of corrosion-resistant steel
12Kh18N10T with a capacity of 20 litres. Air was injected into the supply system by a SO-45A
grade compressor (2), passing through an air absorption dryer (3) HLS-R012-HL0030 entered
an ozoniser (4) "OZON-5PV1" with a power capacity not more than 150 W and a maximum
productivity of 16g/m3. Ozone-resistant PVC hoses and glass tubes were used as a pipeline for
transporting the ozone-air mixture. The ozone content in the air was determined using Medozon
254/5 with a measurement range from 0 to 150 mg/l. The ozone content in the liquid was
measured by Medozon-254/5Zh with a range of measured concentrations from 0.1 to 25 mg/l.
Adjustment of the flow rate of the gas-air mixture was carried out using a rotameter (5) Emis
Meta 210-R-008V-G. As a current source (7), a VSA-5K selenium rectifier was used which
allows adjusting the current in the range from 0 to 20 A at voltages up to 90 V. Contact with
the cathode was carried out using a sliding contact (9). The current strength was monitored with
a high-precision desktop digital multi-meter MS8050. Cooling and mixing were carried out in
a storage tank (12) with the help of a refrigeration unit (8) VS 0.7-3 and a bubbler (6). The
electrolyte with dissolved ozone was fed into the electrolytic bath by a pump (13).
A needle-shaped cathode (10), which is a cylindrical structure with a 40- mm outer diameter
and a 20-mm inner diameter, is made of thin, corrosion-resistant wire with a diameter of 0.1
mm. To ensure uniform contact with the anode plane, the working, end surface of the needle-
shaped cathode was sanded. The rotation speed was provided by an adjustable electric motor
(11). The distance between the electrodes varied in the interval of 0.1-0.5 mm.
Tests were conducted on the D16T alloy. The size of the anode was 60x40x3 mm. The study
of the structure of the samples was carried out using a JEOLJ5M-6150 scanning electron
microscope with an attachment module for X-ray spectral analysis and an Integra Aura atomic-
power probe microscope using the semi-contact method with scanning the sample previously
purified from organic pollutants with ethyl alcohol. Samples were scanned with a resolution of
1024 points per side. When scanning, the relief of the sample and the distribution of the
Figure 2 Scheme of anodizing with a needle-shaped cathode:
1- bath tank; 2- compressor; 3- ozone dryer; 4- ozone generator; 5- rotameter; 6- bubbler; 7-
current source; 8- refrigeration unit; 9- sliding current lead; 10- needle-shaped cathode; 11-
electric motor; 12- storage tank; 13- pump
N. F. Kolenchin
http://www.iaeme.com/IJMET/index.asp 1213 editor@iaeme.com
amplitude and phase of the probe oscillations over the scan area were recorded. The lateral
scanning resolution of the microscope is at least 3 nm, the height resolution is at least 0.5 nm.
Hardness was measured using an ultrasonic contact thickness gauge "Konstanta-K5". This
device allows measuring oxide coatings up to 2 mm thick, excluding preliminary sample
preparation. Hardness was determined by a multifunctional ultrasonic device "Konstanta-
K5U". Measurement limits were from 20 to 80 HRC, error was +/-2. The concentration of
transmitted ozone in the air mixture corresponded to 3 mg/l.
3. RESULTS AND DISCUSSION
The oxidation process was carried out in a 10% aqueous solution of sulfuric acid in the mode
of falling power. Two options were considered — static, when the cathode is stationary, and
mobile, when the cathode is rotating.
When the cathode is stationary, the anode surface located inside the needle-shaped electrode
is partially etched due to insufficient cooling in the domed zone and an increased etching rate
due to the heating of the electrolyte.
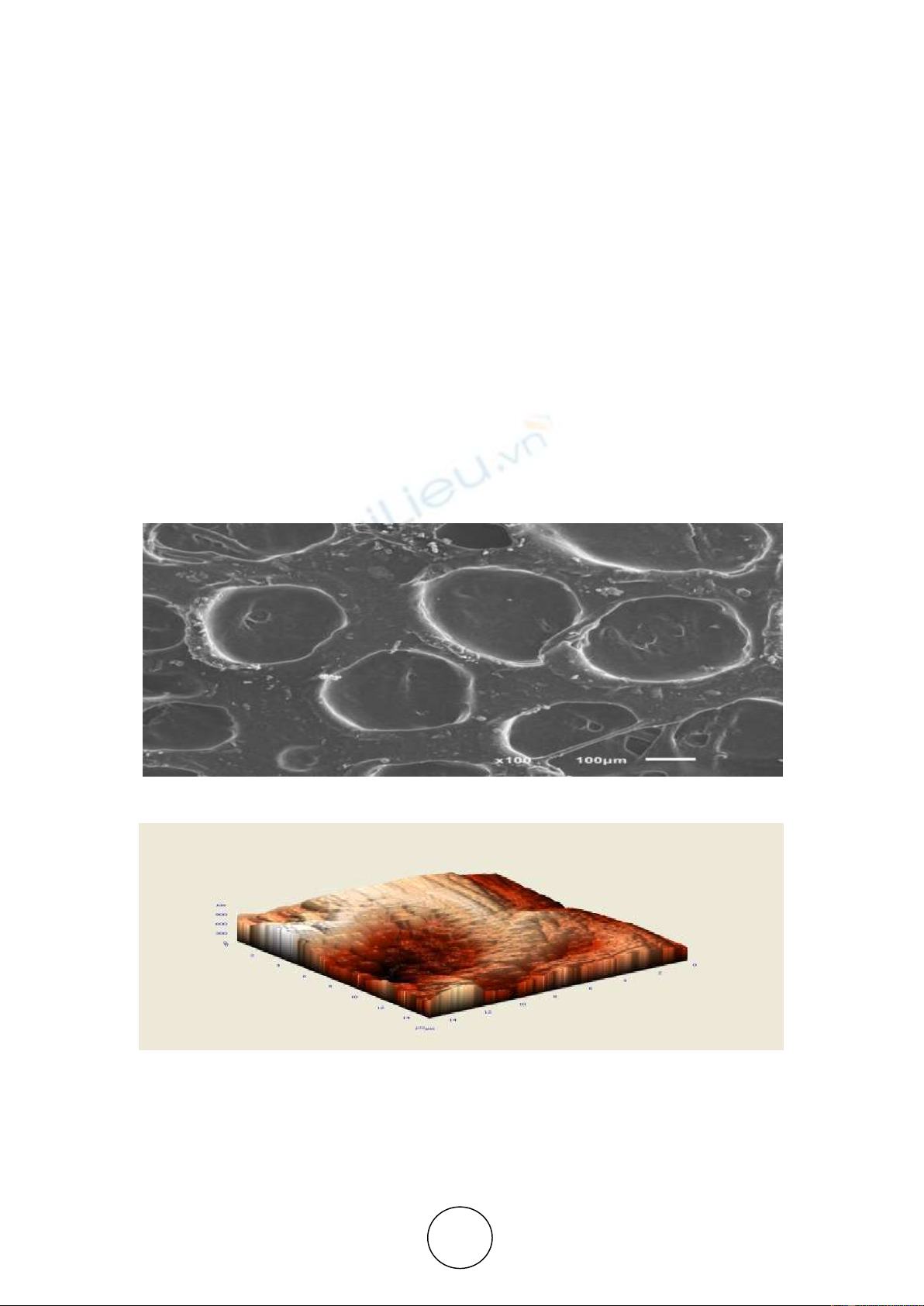
The surface of the anode located opposite the needles was formed with the original texture
presented in Figure 3. a
b
Figure 3 The oxide surface formed with a stationary cathode. Voltage of forming - 25V. Electrolyte - a 10%
solution of sulfuric acid cooled to 0 °C:
а - microgeometry of the flat part of the surface;
b - microgeometry of the surface in 3D
The crater-shaped surface is the result of the concentration of current density on the edges
of the cathode — the centre of elevated temperature and, accordingly, the zone of elevated
The Influence of the Cathode Shape on the Phase Composition and Structure During Oxidation
http://www.iaeme.com/IJMET/index.asp 1214 editor@iaeme.com
etching rates. The film was formed diametrically unevenly in thickness with a decrease in the
direction of the axis of the stationary electrode. Over 60 minutes, the average oxide thickness
turned out to be insignificant and amounted to 15 µm.
By imparting rotation to the cathode at a speed of 150 rpm, the electrolyte bubbling in the
contact zone was improved. The maximum convergence of two electrodes, with intensive
homogenization, provides an increase in current density at the tip, which contributes to the
dissolution of ozone in the interelectrode gap and, accordingly, increases the likelihood of its
participation in oxide formation. The results of measurements of the thickness and hardness of
the oxide layer over 30 minutes of the process are shown in Table 1. The oxide formed on the
inner surface of the sample has low hardness and thickness.
Table 1 Properties of the formed layer with different modes of anodizing
Initial current
density,
A/dm2
Hardness, НRC
Thickness, µm
Along the
contact line
Inside the
contact line
Outside the
contact line
Along the
contact line
Before the
contact line
Outside the
contact line
1
62
41
60
54
32
51
5
66
43
63
57
36
53
10
72
48
68
64
39
57
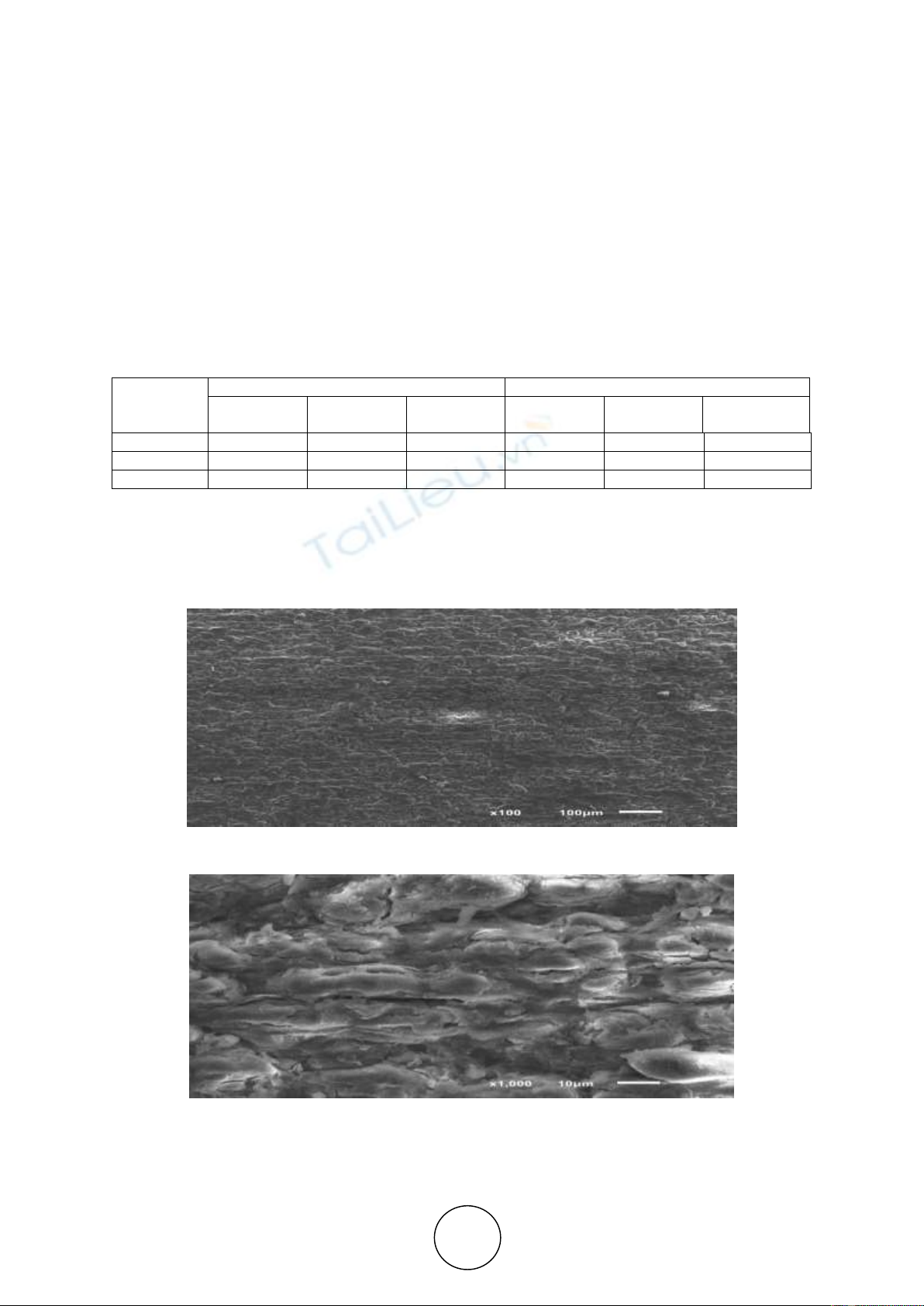
A set of needles is a definite obstacle to the ozonized electrolyte in the inner zone. The strip
under the needle electrode has some advantages in terms of the parameters under study over
anodizing with a flat electrode. On average, the thickness and hardness of the oxide layer were
10% greater.
а
b
Figure 4 Geometry of the pores formed during the rotation of the cathode at a speed of 150 rpm with a
magnification: a-100 times; b-1000 times












![Bài tập tối ưu trong gia công cắt gọt [kèm lời giải chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251129/dinhd8055/135x160/26351764558606.jpg)













