
C-Terminal extension of a plant cysteine protease
modulates proteolytic activity through a partial inhibitory
mechanism
Sruti Dutta, Debi Choudhury, Jiban K. Dattagupta and Sampa Biswas
Crystallography and Molecular Biology Division, Saha Institute of Nuclear Physics, Kolkata, India
Keywords
C-terminal extension; cysteine proteases;
modulation of proteolytic activity;
papain-like; thermostable
Correspondence
S. Biswas, Crystallography and Molecular
Biology Division, Saha Institute of Nuclear
Physics, 1 ⁄AF Bidhannagar, Kolkata
700 064, India
Fax: +91 332 337 4637
Tel: +91 332 337 5345
E-mail: sampa.biswas@saha.ac.in
(Received 14 March 2011, revised 16 May
2011, accepted 22 June 2011)
doi:10.1111/j.1742-4658.2011.08221.x
The amino acid sequence of ervatamin-C, a thermostable cysteine protease
from a tropical plant, revealed an additional 24-amino-acid extension at its
C-terminus (CT). The role of this extension peptide in zymogen activation,
catalytic activity, folding and stability of the protease is reported. For this
study, we expressed two recombinant forms of the protease in Escherichia
coli, one retaining the CT-extension and the other with it truncated. The
enzyme with the extension shows autocatalytic zymogen activation at a
higher pH of 8.0, whereas deletion of the extension results in a more active
form of the enzyme. This CT-extension was not found to be cleaved during
autocatalysis or by limited proteolysis by different external proteases.
Molecular modeling and simulation studies revealed that the CT-extension
blocks some of the substrate-binding unprimed subsites including the speci-
ficity-determining subsite (S2) of the enzyme and thereby partially occludes
accessibility of the substrates to the active site, which also corroborates the
experimental observations. The CT-extension in the model structure shows
tight packing with the catalytic domain of the enzyme, mediated by strong
hydrophobic and H-bond interactions, thus restricting accessibility of its
cleavage sites to the protease itself or to the external proteases. Kinetic
stability analyses (T
50
and t
1⁄2
) and refolding experiments show similar
thermal stability and refolding efficiency for both forms. These data suggest
that the CT-extension has an inhibitory role in the proteolytic activity of
ervatamin-C but does not have a major role either in stabilizing the enzyme
or in its folding mechanism.
Structured digital abstract
lErvC cleaves ErvC by protease assay (View interaction)
ltrypsin cleaves ErvC by protease assay (View interaction)
Introduction
Papain-like cysteine proteases (EC 3.4.22) from plant
sources are of industrial and biotechnological impor-
tance because these enzymes are better suited to various
industrial processes [1]. A cysteine protease is expressed
as an inactive precursor in a pre-proenzyme form
which contains a signal peptide (pre-), an inhibitory
Abbreviations
CT, C-terminal; E-64, 1-[L-N-(trans-epoxysuccinyl)leucyl]amino-4-guanidinobutane; Erv-C, ervatamin-C; pNA, p-nitroanilide; rmErv-C
+CT
,
recombinant mature ervatamin-C with C-terminal extension; rmErv-C
DCT
, recombinant mature ervatamin-C without C-terminal extension;
rproErv-C
+CT
, recombinant proervatamin-C with C-terminal extension; rproErv-C
DCT
, recombinant proervatamin-C without C-terminal
extension.
3012 FEBS Journal 278 (2011) 3012–3024 ª2011 The Authors Journal compilation ª2011 FEBS

pro-region and a mature catalytic domain [2–4]. Fol-
lowing synthesis, the pre-peptide is removed during
passage to the lumen of the endoplasmic reticulum [2].
The inactive proenzyme subsequently undergoes prote-
olytic processing to produce an active mature enzyme
by autocatalytic cleavage of the propeptide part at the
N-terminus [2]. It is known that the propeptide at the
N-terminus of the protease acts as an intramolecular
chaperone to mediate correct folding of the protease
[2]. The mature catalytic domain of the enzyme of this
family has a molecular mass of 21–30 kDa and
shares a common fold with papain, the archetype
enzyme of the family. These proteases are folded into
two compact interacting domains of comparable size,
delimiting a cleft which contains the active site residues
cysteine and histidine, forming a zwitterionic catalytic
dyad (Cys
)
...His
+
) [5].
Sometimes a larger precursor is also synthesized
which contains a C-terminal (CT) extension ⁄propep-
tide in addition to the abovementioned N-terminal
propeptide flanking the mature protease domain [6,7].
Unlike N-terminal propeptides, the role of CT-exten-
sions (or propeptides) is not yet well established.
Sometimes an endoplasmic reticulum retention signal
Lys-Asp-Glu-Leu (KDEL) is found in the CT-propep-
tide which regulates the delivery of protease precursor
to other cellular compartments [8]. Some other mem-
bers of the papain family from plant sources also con-
tain a larger CT-propeptide domain which shares a
similarity with animal epithelin ⁄granulin and the func-
tion of this domain is reported to be involved in leaf
senescence [6]. In addition, a CT-propeptide without
any specific domain or motif is observed in some
papain-like cysteine proteases from plants like Nicoti-
ana tabacam (Q84YH7), Actinidia chinensis (P00785)
and Vicia sativa (Q41696). In most of the cases, such a
CT-extension contains the vacuolar sorting signal and
is cleaved inter- or intramolecularly after sorting [9].
No conserved sequence motif has been found in the
vacuolar sorting signal at CT-propeptide, rather an
amphipathic-like (hydrophobic and acidic) motif is
generally observed [9,10] at the core of such peptides.
Other than plant systems, a CT-extension found in
a lysosomal cysteine protease (Lpcys2) of Leish-
mania pifanoi, plays a role in the regulation of enzyme
activity [11]. CT-extension in mammalian and yeast
bleomycin hydrolase [12,13] is a key factor which regu-
lates their endo-peptidase or exo-aminopepetidase
activity by blocking the unprimed subsites in the
enzymes.
Ervatamin-C (Erv-C) is a papain-like cysteine prote-
ase (EC 3.4.22) with high stability purified from the
latex of a tropical plant Ervatamia coronaria [14]. The
3D structure of Erv-C reveals an extra disulfide bond,
shorter loop regions and additional electrostatic inter-
actions in the interdomain space, which are thought to
be responsible for its high stability [15]. Sequencing
of the cDNA (from mRNA) of Erv-C from the leaf
of the plant in our laboratory [16], and comparison of
the cDNA-derived amino acid sequence with other
members of the family reveal that Erv-C is synthesized
as a precursor protein and in addition to the pre- (19
amino acids), pro- (114 amino acids) and mature (208
amino acids) parts, it contains an extension of 24
amino acids at the CT of the mature enzyme [16]
(Fig. 1A). This CT-extension was not observed, how-
ever, when the mature Erv-C was purified directly
from the latex of the plant [15].
In this article, we attempt to understand the role of
this CT-extension in zymogen activation, enzyme activ-
ity, folding and stability in vitro at the molecular level
from structural and functional points of view.
Results
Cloning, expression, purification and refolding of
rproErv-C
DCT
and rproErv-C
+CT
Both the proteins, recombinant proervatamin-C with-
out the CT-extension (rproErv-C
DCT
) and recombinant
proervatamin-C with the CT-extension (rproErv-
C
+CT
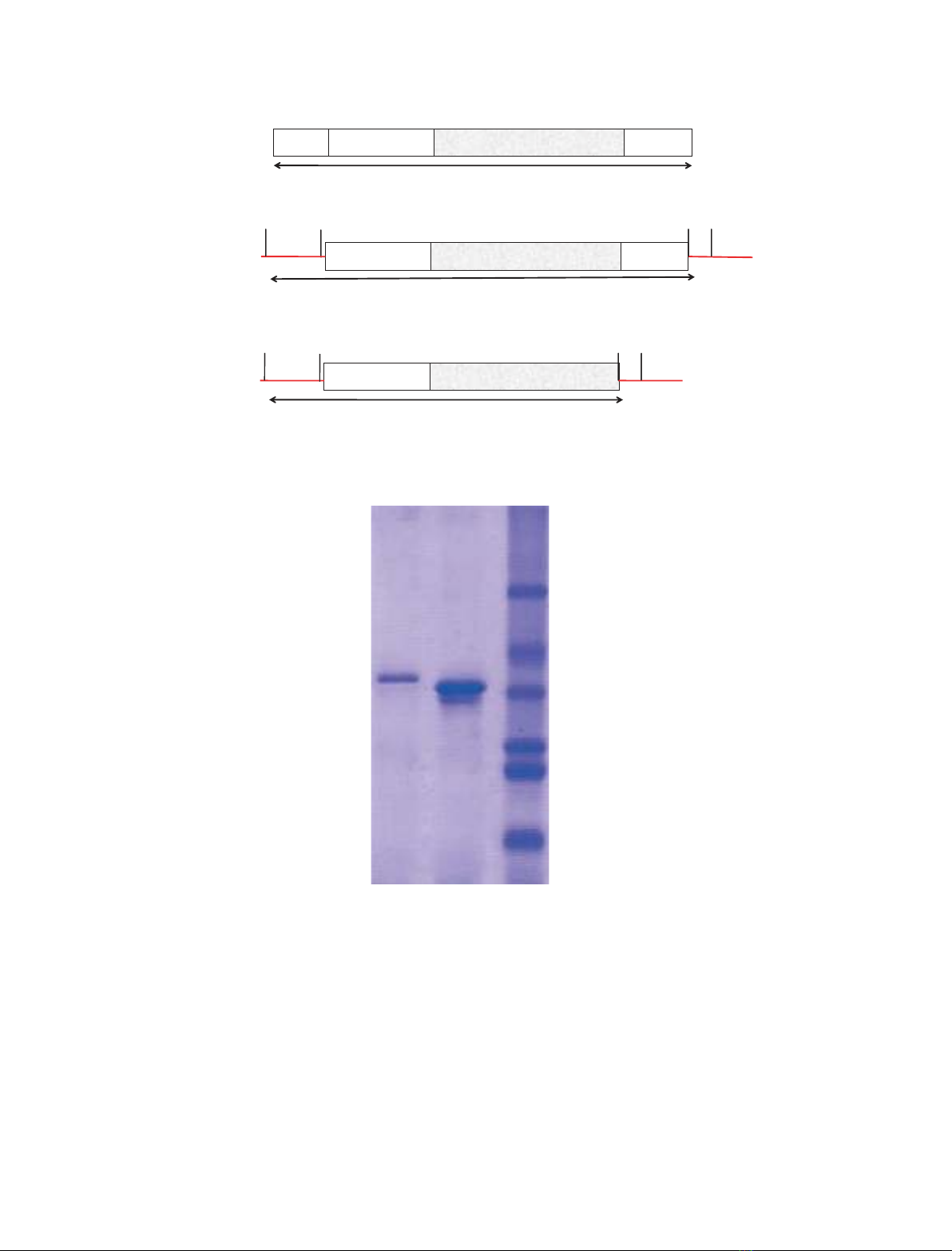
), were expressed in E. coli as inclusion bodies
with an apparent molecular mass of 41 and
43 kDa (Fig. 1B), respectively, which is consistent
with the estimated molecular masses of their deduced
amino acid sequences. Correct refolding was checked
by gelatin gel assay. The condition and efficiency of
refolding were almost similar for both forms with
> 90% recovery of the folded form from Ni-NTA
purified protein for each (Table S1).
Activation to mature protease
The purified refolded rproErv-C
+CT
could be con-
verted into its mature active form (rmErv-C
+CT
)by
using cysteine (20 mM) as the activator in 50 mMTris
buffer, pH 8.00, at 60 C for 25–30 min, whereas the
purified refolded rproErv-C
DCT
could be converted
into its mature form (rmErv-C
DCT
) by the same activa-
tor in 50 mMNa-acetate buffer, pH 4.5, at 60 C for
45 min (Fig. 2A). Thus the zymogen activation process
occurs at different pH and time of maturation for the
two enzymes. The molecular mass of the mature
enzyme rmErv-C
+CT
is higher (27 kDa) than that
of rmErv-C
DCT
(25 kDa) as observed in the
SDS ⁄PAGE analyses (Fig. 2A). This difference in
S. Dutta et al. Role of C-terminal extension in a cysteine protease
FEBS Journal 278 (2011) 3012–3024 ª2011 The Authors Journal compilation ª2011 FEBS 3013
molecular mass almost fits the theoretically calculated
value for the 24-amino-acid CT-extension. We
expected, therefore, that the CT-extension continued to
remain attached with the mature enzyme even after
autocatalytic processing of rproErv-C
+CT
. Gelatin gel
assay (Fig. 2B) and western blot analyses (Fig. 2C)
also confirmed the retention of the CT-extension in the
mature rmErv-C
+CT
.
Because Erv-C isolated from the latex of the same
plant does not show the CT-extension, the possibility
19 aa 114 aa 208 aa 24 aa
Pre N-Pro Protease domain CT - ex
365 aa
H1
I
NPro CT ex
114 aa 208 aa 24 aa
BamH
34 aa
Start
Stop
Xho1
N-Pro Protease domain
Protease domain
CT - ex
380 aa
1
II
NP
114 aa 208 aa
BamH1
34 aa
Start
Stop
Xho1
SS SS G
SSS
S
N-Pro
356 aa
III
MSTLFIISILLFLASFSYAMDISTIEYKYDKSSAWRTDEEVKEIYELWLAKHDKVYSGLVEYEKRFEIFKDNLKFIDEH
NSENHTYKMGLTPYTDLTNEEFQAIYLGTRSDTIHRLKRTINISERYAYEAGDNLPEQIDWRKKGAVTPVKNQGKCG
SCWAFSTVSTVESINQIRTGNLISLSEQQLVDCNKKNHGCKGGAFVYAYQYIIDNGGIDTEANYPYKAVQGPCRAAK
KVVRIDGYKGVPHCNENALKKAVASQPSVVAIDASSKQFQHYKSGIFSGPCGTKLNHGVVIVGYWKDYWIVRNSW
GRYWGEQGYIRMKRVGGCGLCGIARLPYYPTKAAGDENSKLETPELLQWSEEAFPLA
IV
A
B
66 kDa
45 kDa
36 kDa
29 kDa
24 kDa
20 kDa
321
Fig. 1. (A) (I) Open reading frame of Erv-C precursor, pre-pro-ErvC. (II) Recombinant ervatamin-C with C-terminal extension, rproErv-C
+CT
.
(III) rproErv-C
DCT
, recombinant ervatamin-C without C-terminal extension. Red indicates vector portion and ‘aa’ stands for amino acids. (IV)
The amino acid sequence of the open reading frame. The sequence of CT-extension is in red. (B) Lanes 1 and 2, Purified proteins rproErv-
C
+CT
and rproErv-C
DCT
, respectively; lane 3, Molecular mass markers.
Role of C-terminal extension in a cysteine protease S. Dutta et al.
3014 FEBS Journal 278 (2011) 3012–3024 ª2011 The Authors Journal compilation ª2011 FEBS

of removal of the CT-extension by other plant prote-
ases in vivo could not be ruled out. To explore this
possibility, we performed a trans mode activation of
rproErv-C
+CT
in vitro using seven different proteases
(four cysteine proteases Erv-A, -B, -C and papain from
the plant latex; two serine proteases trypsin and chy-
motrypsin from bovine pancreas; one aspartic protease
pepsin from porcine stomach mucosa), each having
sequences specific for their cleavage in the amino acid
sequence of the CT-extension. The activated mature
protein thus generated in each case shows a band at
the same position (27 kDa) like that in the autoacti-
vated enzyme, as observed in SDS ⁄PAGE analyses
(Fig. S1), indicating that these external enzymes can
not cleave the CT-extension. Even with a prolonged
digestion time (24 h), the same result was obtained for
all proteases except trypsin (Fig. S1). Trypsin digestion
for 24 h resulted in a truncated protein at 26 kDa,
slightly above the activated mature rmErv-C
DCT
(25 kDa). This result probably indicates that trypsin
has some accessibility to its sites of specificity (Lys,
which is in position 7 of the CT-extension) (Fig. S2)
and only after a prolonged incubation time can it
result in a band at a slightly lower molecular mass
position, as observed in the SDS ⁄PAGE analysis
(Fig. S1).
Specific activity and optimum temperature of
activity
The optimum temperature of activity, T
opt
(Fig. 3), for
both forms is 65 C. Interestingly, it was observed that
rproErv-C
+CT
shows no activity below 45 C and then
activity rises sharply from 60 C onwards, reaching a
maximum at 65 C. In the case of rproErv-C
DCT
,a
gradual increase in activity with temperature was
observed until it reached its maximum at 65 C.
At T
opt
(65 C), the specific activity of rmErv-C
DCT
was found to be almost double that of rmErv-C
+CT
(Table 1). At 37 C, however, rproErv-C
DCT
shows
measurable proteolytic activity, whereas no activity
was seen for rmErv-C
+CT
.
Kinetic measurements of the recombinant
proteins
Kinetic constants of rproErv-C
+CT
and rproErv-C
DCT
were measured at room temperature against N-ben-
zoyl-Phe-Val-Arg-p-nitroanilide (pNA), a tripeptide
substrate with a valine at the P2 position which is
known to act as a substrate for Erv-C [17]. The kinetic
constants of the two recombinant enzymes (Table 1)
clearly show that rproErv-C
DCT
has almost 10 times
97 kDa
66 kDa
66 kDa
45 kDa
36 kDa
29 kDa
24 kDa
20 kDa
43 kDa rproErv-C+CT
rproErv-C+CT
rproErv-C∆CT
rmErv-C+CT
rmErv-C∆CT
rproErv-C∆CT
rmErv-C∆CT
rmErv-C+CT
29 kDa
20 kDa
M
1123
2
45 30 20 10 0 C25 15 5 M4530 20 10 0 C25 15 5
A
BC
Fig. 2. (A) Time course of activation to the mature form. (I) rproErv-C
+CT
. (II) rproErv-C
DCT
as discussed in Materials and methods. Time
intervals of 0–45 min are indicated for the respective lanes. Untreated proteins (control) are labelled as ‘C’, ‘M’ denotes the molecular mass
marker. (B) Gelatin gel assay of activated rproErv-C
DCT
(lane 1) and rproErv-C
+CT
(lane 2). (C) Western blot analysis. Lane 1, Erv-C purified
from the plant latex; lanes 2 and 3, purified and refolded rproErv-C
+CT
and rproErv-C
DCT
. [Correction added on 26 July 2011 after original
online publication: in the figure, labelling for part C was changed from ‘rproErv-C
+CT
, rproErv-C
DCT
, rproErv-C
+CT
and rproErv-C
DCT
to rproErv-C
+CT
,
rproErv-C
DCT
, rmErv-C
+CT
and rmErv-C
DCT
’].
S. Dutta et al. Role of C-terminal extension in a cysteine protease
FEBS Journal 278 (2011) 3012–3024 ª2011 The Authors Journal compilation ª2011 FEBS 3015
higher activity than rproErv-C
+CT
. One can probably
conclude that the CT-extension has some inhibitory
effect on the activity of the enzyme against a small
peptide.
Thermal stability
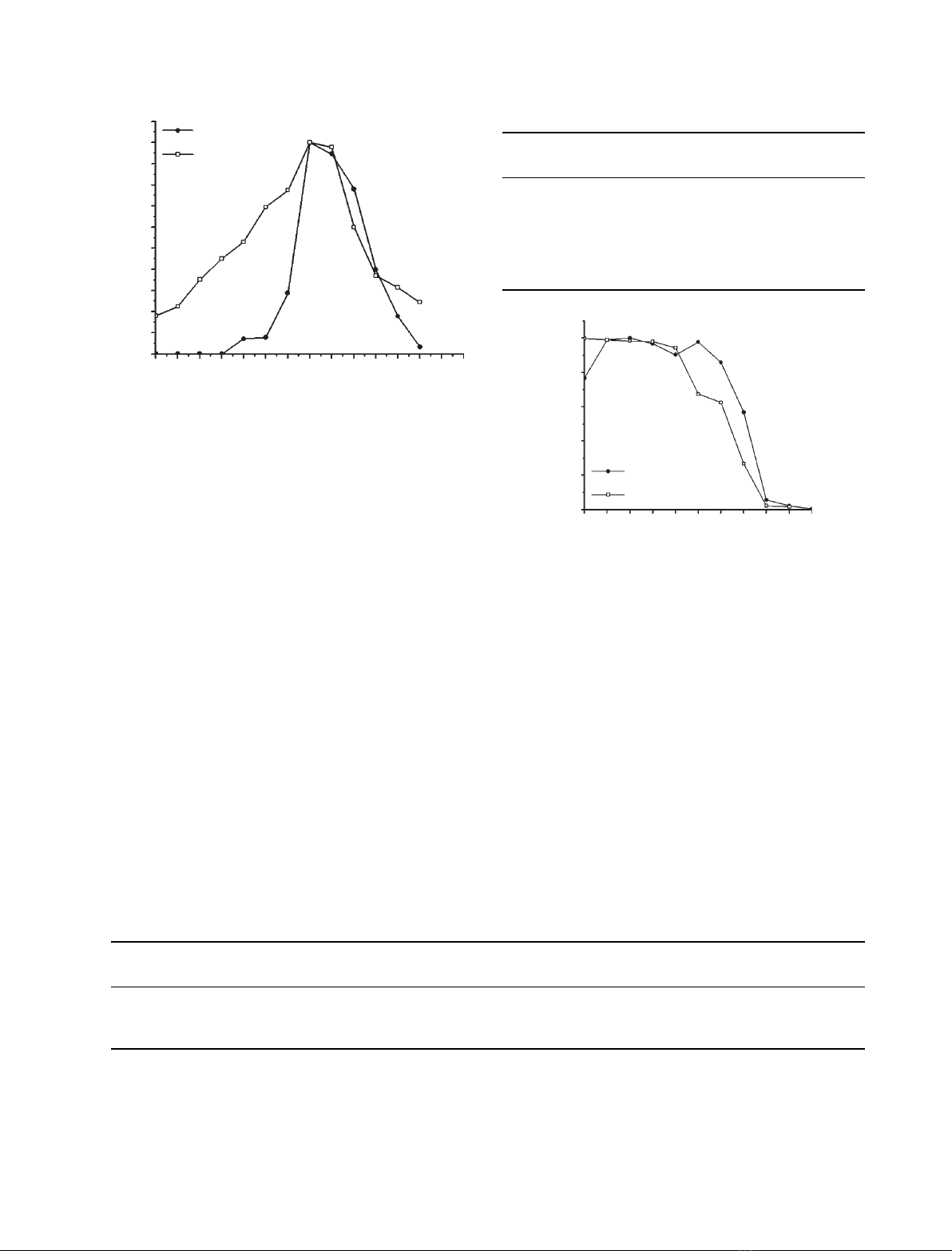
Temperatures of maximum proteolytic activity (T
max
)
for rproErv-C
+CT
and rproErv-C
DCT
are 50 and 45 C
(Table 2 and Fig. 4) and they retain > 90% activity
up to 65 and 60 C, respectively. These data indicate a
good thermotolerance for these enzymes. The pattern
of retention and fall in activity beyond T
max
was more
or less the same for both enzymes. The T
50
values for
rproErv-C
+CT
and rproErv-C
DCT
were 76 and 72 C
(Fig. 4), respectively. The half lives (t
1⁄2
)at65C were
400 min (Fig. 5) for both enzymes.
Molecular modeling studies
To gain insight into the stability and dynamic proper-
ties of the structure, solvent MD simulation was
110
100
rproErv-C+CT
rproErv-C∆CT
90
80
70
60
Residual activity (%)
Temperature (°C)
50
40
30
20
10
0
30 35 40 45 50 55 60 65 70 75 80 85 90 10095
Fig. 3. Determination of optimum temperature of activity (T
opt
)of
rproErv-C
+CT
and rproErv-C
DCT
. Purified proenzymes (10–20 lg)
were converted to their respective mature forms and the percent-
age residual enzyme activities were determined with respect to the
maximum activity using an azocasein assay at different tempera-
tures, as described in Materials and methods. Each data point is an
average of three independent experiments having similar values
(Table S3).
Table 1. Kinetic constants using the substrate N-benzoyl-Phe-Val-Arg-pNA. Specific activity using azocaesin and IC
50
value for the inhibitor
E-64. ND, not determined.
k
cat
(s
)1
)
a
K
m
(lM)
a
k
cat
⁄K
m
(s
)1
ÆmM
)1
)
Specific activity
at 37 C
b
(UÆmg
)1
)
Specific activity
at 65 C
b
(UÆmg
)1
)
IC
50
against
E-64 (nM)
c
rproErv-C
+CT
0.0170 ± 0.004 88.33 ± 56.77 0.193 No activity 15.87 ± 1.36 482.5 ± 108.0
rproErv-C
DCT
0.2295 ± 0.057 127.3 ± 48.46 1.803 13.21 ± 2.32 35.27 ± 1.78 349.1 ± 62.0
Latex Erv-C [17] 9.312 1063 8.76 75.0 ND 225.0
a
Given standard errors were calculated based on nonlinear fitting of the Michaelis–Menten saturation curve using the software Graphpad
PRISM (http://www.graphpad.com/prism).
b
Each value of specific activity of rproErv-C+CT and rproErv-CDCT is a mean of three independent
experiments ± SD.
c
Given standard deviations were calculated from linear regression plot of residual activity and inhibitor concentration.
Table 2. Kinetic stabilities. ND, not determined.
T
max
(C) T
50
(C)
t
1⁄2
at
65 C (min) T
opt
(C)
rproErv-C
+CT
(activity at 65 C)
50 76 400 65
rproErv-C
DC
(activity at 65 C)
45 72 400 65–70
Native mature Erv-C
(latex) [14,32]
70 76 ND 50
100
rproErv-C+CT
rproErv-C∆CT
80
60
Residual activity (%)
Temperature (°C)
40
20
0
40 45 50 55 60 65 70 75 80 85 90
Fig. 4. Effect of temperature on activity of rproErv-C
+CT
and rpr-
oErv-C
DCT
. Each purified proenzyme (10–20 lg) was treated for
10 min at different temperatures followed by activation of the pro-
proteins to their respective mature forms. The percentage residual
enzyme activities (at each temperature) were determined with
respect to the maximum activity using an azocasein assay at 65 C
as described in Materials and methods. Each data point is an aver-
age of three independent experiments having similar values
(Table S3).
Role of C-terminal extension in a cysteine protease S. Dutta et al.
3016 FEBS Journal 278 (2011) 3012–3024 ª2011 The Authors Journal compilation ª2011 FEBS


























