
Identification and characterization of two dipeptidyl-
peptidase III isoforms in Drosophila melanogaster
Claire Mazzocco
1
, Jennifer Gillibert-Duplantier
2
, Veronique Neaud
2
, Kayoko M. Fukasawa
3
,
Ste
´phane Claverol
4
, Marc Bonneu
4
and Jacques Puiroux
1
1 Laboratoire de Neurobiologie des Re
´seaux, CNRS-UMR 5816, Universite
´Bordeaux I, Talence, France
2 Groupe de Recherche pour l’Etude du Foie, INSERM E9917, Universite
´Victor Segalen Bordeaux II, Bordeaux, France
3 Department of Oral Biochemistry, Matsumoto Dental College, Nagano, Japan
4 Plateforme Ge
´nomique Fonctionnelle, Universite
´Victor Segalen Bordeaux II, Bordeaux, France
Dipeptidyl-peptidase (DPP) III (EC 3.4.14.4) has been
characterized in rat [1,2] and human [3] as a soluble
enzyme (molecular mass 82 kDa, SwissProt accession
numbers O55096 and Q9NY33-1), confirming the
results of their cloning and sequencing. This zinc met-
allopeptidase has also been reported to contain a speci-
fic HELLGH domain [4] which cleaves the second
bound peptide of enkephalins.
Current functional analyses of genomes have allowed
the identification of putative DPP IIIs in about 20
species. In most cases, they are deduced as 700-amino
acid proteins containing the specific catalytic motif
HELLGH-52X-E. However, in a few cases, DPP IIIs
have been predicted despite the shorter presumed
DPP III and ⁄or the lack of the specific HELLGH
domain. In particular, the HELLGH domain is missing
from the hypothetical Caenorhabditis elegans DPP III
(NP492288, 682 residues). In humans, a truncated 317-
residue DPP III isoform (SwissProt accession number
Q9NY33-2), lacking 420 amino acids including the
Keywords
dipeptidyl-peptidase III; enkephalinase;
neuropeptide; proctolin; proteomic
Correspondence
J. Puiroux, Laboratoire de Neurobiologie des
Re
´seaux, CNRS-UMR 5816, Universite
´
Bordeaux I, Avenue des Faculte
´s,
33405 Talence Cedex, France
Fax: +33 540 002561
Tel: +33 540 002569
E-mail: j.puiroux@lnr.u-bordeaux1.fr
(Received 2 November 2005, revised
4 January 2006, accepted 9 January 2006)
doi:10.1111/j.1742-4658.2006.05132.x
Dipeptidyl-peptidase III (DPP III) hydrolyses small peptides with a broad
substrate specificity. It is thought to be involved in a major degradation
pathway of the insect neuropeptide proctolin. We report the purification
and characterization of a soluble DPP III from 40 g Drosophila melanogas-
ter. Western blot analysis with anti-(DPP III) serum revealed the purifica-
tion of two proteins of molecular mass 89 and 82 kDa. MS ⁄MS analysis of
these proteins resulted in the sequencing of 45 and 41 peptide fragments,
respectively, confirming 60% of both annotated D. melanogaster DPP III
isoforms (CG7415-PC and CG7415-PB) predicted at 89 and 82 kDa.
Sequencing also revealed the specific catalytic domain HELLGH in both
isoforms, indicating that they are both effective in degrading small pep-
tides. In addition, with a probe specific for D. melanogaster DPP III, nor-
thern blot analysis of fruit fly total RNA showed two transcripts at 2.6
and 2.3 kb, consistent with the translation of 89-kDa and 82-kDa DPP III
proteins. Moreover, the purified enzyme hydrolyzed the insect neuropeptide
proctolin (K
m
4lm) at the second N-terminal peptide bound, and
was inhibited by the specific DPP III inhibitor tynorphin. Finally, anti-
(DPP III) immunoreactivity was observed in the central nervous system of
D. melanogaster larva, supporting a functional role for DPP III in procto-
lin degradation. This study shows that DPP III is in actuality synthesized
in D. melanogaster as 89-kDa and 82-kDa isoforms, representing two
native proteins translated from two alternative mRNA transcripts.
Abbreviations
DPP III, dipeptidyl-peptidase III.
1056 FEBS Journal 273 (2006) 1056–1064 ª2006 The Authors Journal compilation ª2006 FEBS

catalytic motif HELLGH-52X-E, has also been deduced
in addition to the long isoform. The DPP activity of
such proteins is always questionable until they have
been expressed and purified and their activity tested.
Regardless of the authenticity of the short human
DPP III, it is worth noting the presence of two DPP III
isoforms in this species. In a slightly different fashion,
two DPP III isoforms of 80 and 76 kDa have been iden-
tified in the cockroach Blaberus craniifer [5]. Thereafter,
an ORF corresponding to a 723-residue DPP III
(82 kDa) was deduced in Drosophila melanogaster [5],
and a single gene encoding DPP III was annotated in
the D. melanogaster genome as CG7415 [6]. This con-
trasted with the detection of 89-kDa and 82-kDa protein
bands in this insect [5] and in S2 cells stably transfected
with a CG7415-related cDNA clone [7]. The synthesis of
a longer putative DPP III of 786 amino acids (89 kDa)
in D. melanogaster was finally speculated to result from
alternative splicing of mRNA. In these conditions, the
expression of two putative DPP III isoforms at 89 and
82 kDa is consistent with the prediction of the two
DPP III proteins in D. melanogaster (786 and 723
amino acids) deduced from cDNA clones.
Gathering information from proteomic analysis is
at present necessary to validate gene annotation [8].
Unusually, the analysis of the D. melanogaster genome
suggested that DPP III is probably expressed as two
long isoforms, both including the catalytic domain
HELLGH required for enzyme activity, but this nee-
ded to be validated. Therefore, we here describe the
characterization of two DPP III proteins actually syn-
thesized in D. melanogaster as native 89-kDa and
82-kDa isoforms. Moreover, northern blot analysis
revealed two DPP III mRNA transcripts with the
expected sizes for the distinct translations of the two
isoforms. Substrate specificity and inhibition studies
confirmed that the purified enzyme displayed the cru-
cial features of DPP III. In addition, we report the
localization of DPP III immunoreactivity in the central
nervous system of D. melanogaster.
Results
Purification of soluble fruit fly DPP III
D. melanogaster soluble DPP III was purified by chro-
matography by the method used to purify putative
D. melanogaster DPP III functionally expressed in S2
cells [7] and described in Experimental procedures. The
overall recovery of DPP III activity, as verified by
reversed-phase chromatography of met-enkephalin
degradation products produced during incubation with
a fraction aliquot, was 7% and corresponded to a
46-fold purification of about 400 lg protein. Analysis
of pooled active fractions by SDS ⁄PAGE and western
blotting with an antibody to rat liver DPP III revealed
only two protein bands, at 89 and 82 kDa (Fig. 1A,
lanes 1 and 2).
MS/MS analysis of purified enzyme
Purified DPP of fruit fly (6 lg) was electrophoresed on
a 10% polyacrylamide slab gel. After Coomassie Bril-
lant Blue staining, the protein bands at 89 and 82 kDa
were excised and processed for sequence analysis. After
two separate trypsin digestions and reversed-phase sep-
arations, 41 peptide fragments (437 amino acids) were
sequenced from the 82-kDa protein band and 45 pep-
tide fragments (483 amino acids) were sequenced from
the 89-kDa protein band, including the 41 sequences
previously mentioned. The four fragments (46 amino
acids) specifically sequenced from the 89-kDa protein
band were found along the N-terminal region of the
786-residue D. melanogaster DPP III (SwissProt No
Q9VHR8-1), and matched 46 out of the first 63 amino
acids with 100% identity. The remaining 41 sequenced
A
B
Fig. 1. SDS ⁄PAGE and western blot of purified D. melanogaster
DPP III and northern blot analysis of D. melanogaster DPP III tran-
scripts. (A) 10-lL aliquots of pooled active fractions obtained from
Superdex chromatography were separated on SDS ⁄PAGE and sil-
ver-stained (lane1) or analysed by western blotting with DPP III
antibody (lane 2). Two major bands at 89 and 82 kDa (left arrows)
were identified with both methods. Molecular mass markers are
indicated in kDa on the right. (B) Total RNA was extracted from
fruit flies and fractionated on a 1.5% agarose gel, blotted to a
Hybond N
+
membrane and hybridized using Ultrahyb solution with
the a
32
P-labeled D. melanogaster DPP III PCR probe (783 bp). Two
transcripts were visualized at 2.3 and 2.6 kb (right arrows). RNA
molecular mass markers are indicated in kilobase pairs on the left.
C. Mazzocco et al.Two DPP III isoforms in the fruit fly
FEBS Journal 273 (2006) 1056–1064 ª2006 The Authors Journal compilation ª2006 FEBS 1057
peptides were distributed in the protein region between
amino acids 64 and 786, representing the 723-residue
long DPP III isoform (SwissProt No Q9VHR8-2),
starting at the methionine at position 64 of the longest
D. melanogaster DPP III. Of the 45 sequences avail-
able, almost 62% of the long D. melanogaster DPP III
isoform were identified and almost 61% of the shorter
conceptual DPP III protein were thus verified.
Furthermore, MS ⁄MS suggested that none of the
sequenced peptide fragments displayed either glycosy-
lation or phosphorylation. For instance, four N-X-T
or N-X-S potential glycosylation sites out of six were
recovered as nonglycosylated among the sequenced
fragments of D. melanogaster DPP III. Finally, the
catalytic site HELLGH-52X-E was found among the
sequenced fragments of both D. melanogaster DPP III
isoforms, corroborating the DPP III activity measured
in the purified enzyme. In addition, after northern blot
analysis of fruit fly total RNA using a specific D. mel-
anogaster DPP III probe (783 bp), two bands at 2.6
and 2.3 kb were revealed (Fig. 1B).
Activity assay, kinetic studies and inhibition
stimulation of the purified enzyme
The purified D. melanogaster enzyme efficiently hydro-
lysed proctolin (40 lm) over 1 h incubation as indica-
ted by reversed-phase separation of the degradation
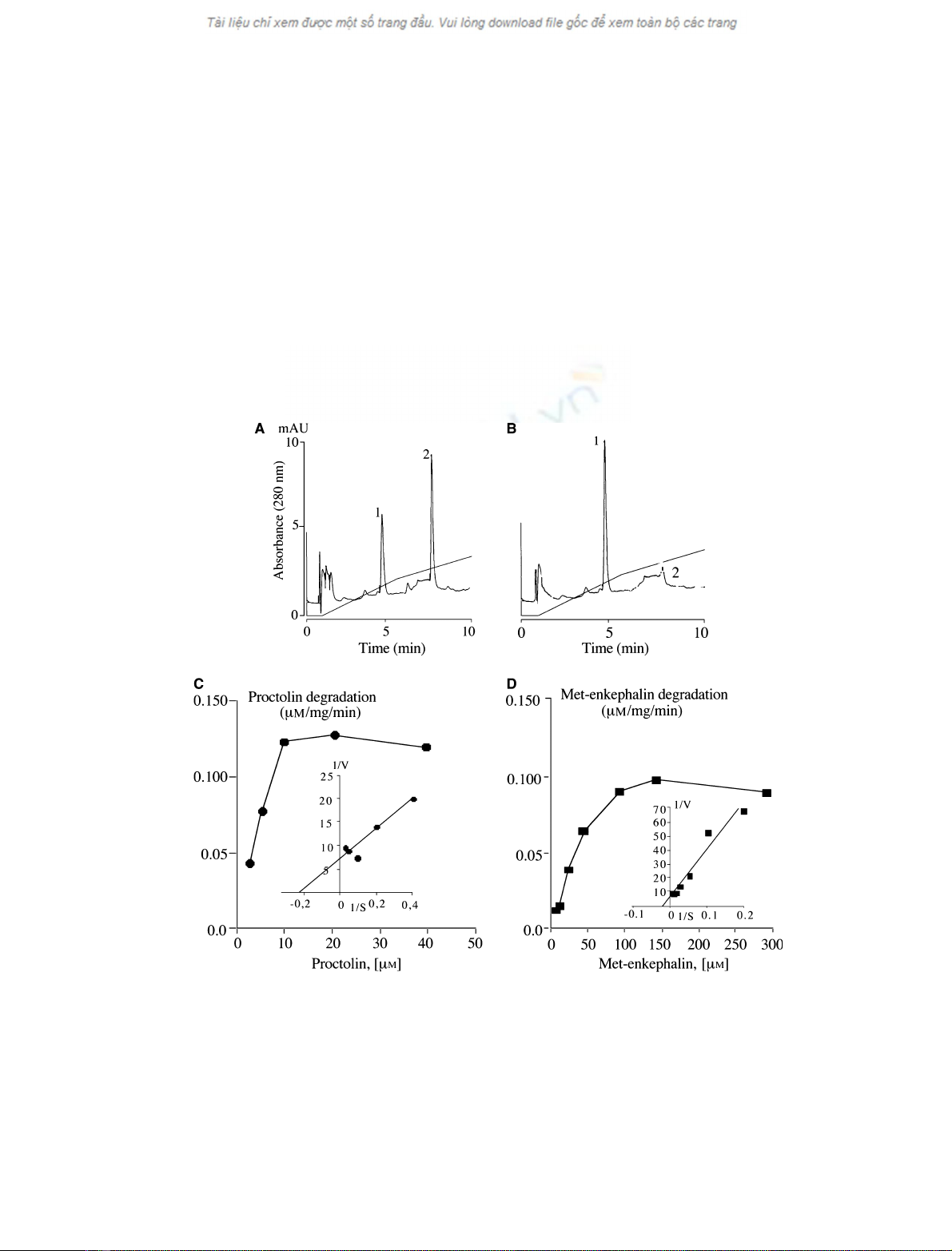
Fig. 2. Degrading activity and kinetic studies of purified D. melanogaster DPP III. (A,B) Elution profiles (280 nm) obtained after reversed-
phase separation of samples prepared from proctolin (20 lM) incubated with purified D. melanogaster DPP III for 15 min (A) and 60 min (B).
Proctolin (peak 2) was eluted at 8 min, and only traces are detected after 1 h incubation. The N-terminal dipeptide Arg-Tyr (peak 1) was
eluted at 5 min and significantly increased according to the duration of incubation. (C,D) Increasing concentrations of proctolin (C; 2.5–
40 lM) and met-enkephalin (D; 5–300 lM) were incubated for 30 min with purified D. melanogaster DPP III. The samples were separated by
reversed-phase chromatography to identify and quantify the remaining proctolin or met-enkephalin and their metabolites produced during
incubation. The results of saturation are the mean of three independent measurements and are expressed as lmol neuropeptide degra-
dedÆmin
)1
Æ(mg purified enzyme)
)1
(corresponding Lineweaver–Burk plots are included).
Two DPP III isoforms in the fruit fly C. Mazzocco et al.
1058 FEBS Journal 273 (2006) 1056–1064 ª2006 The Authors Journal compilation ª2006 FEBS
products. Only the N-terminal dipeptide Arg-Tyr
(monitored at 280 nm, Fig. 2A,B, and 206 nm,
Fig. 3A,C) and the C-terminal tripeptide Leu-Pro-Thr
(detected at 206 nm, Fig. 3A,C) were liberated from
proctolin incubated with the purified enzyme, in the
presence of bestatin (100 lm) to inhibit aminopepti-
dase activity. After a 2 h incubation, no other degrada-
tion products could be detected other than the
dipeptide Arg-Tyr (Fig. 2B) and the tripeptide (not
shown). We further characterized the degrading activ-
ity of the purified D. melanogaster enzyme by incuba-
tion with increasing concentrations of the insect
neuropeptide proctolin (from 2.5 to 40 lm) and met-
enkephalin (from 5 to 300 lm). A K
m
of 4.5 lmwas
calculated for proctolin (Fig. 2C) and 41.9 lmfor
met-enkephalin (Fig. 2D). V
max
values were 0.14 lmol
proctolinÆmin
)1
Æ(mg protein)
)1
and 0.11 lmol met-
enkephalinÆmin
)1
Æ(mg protein)
)1
.
Compared with the rate of proctolin degradation in
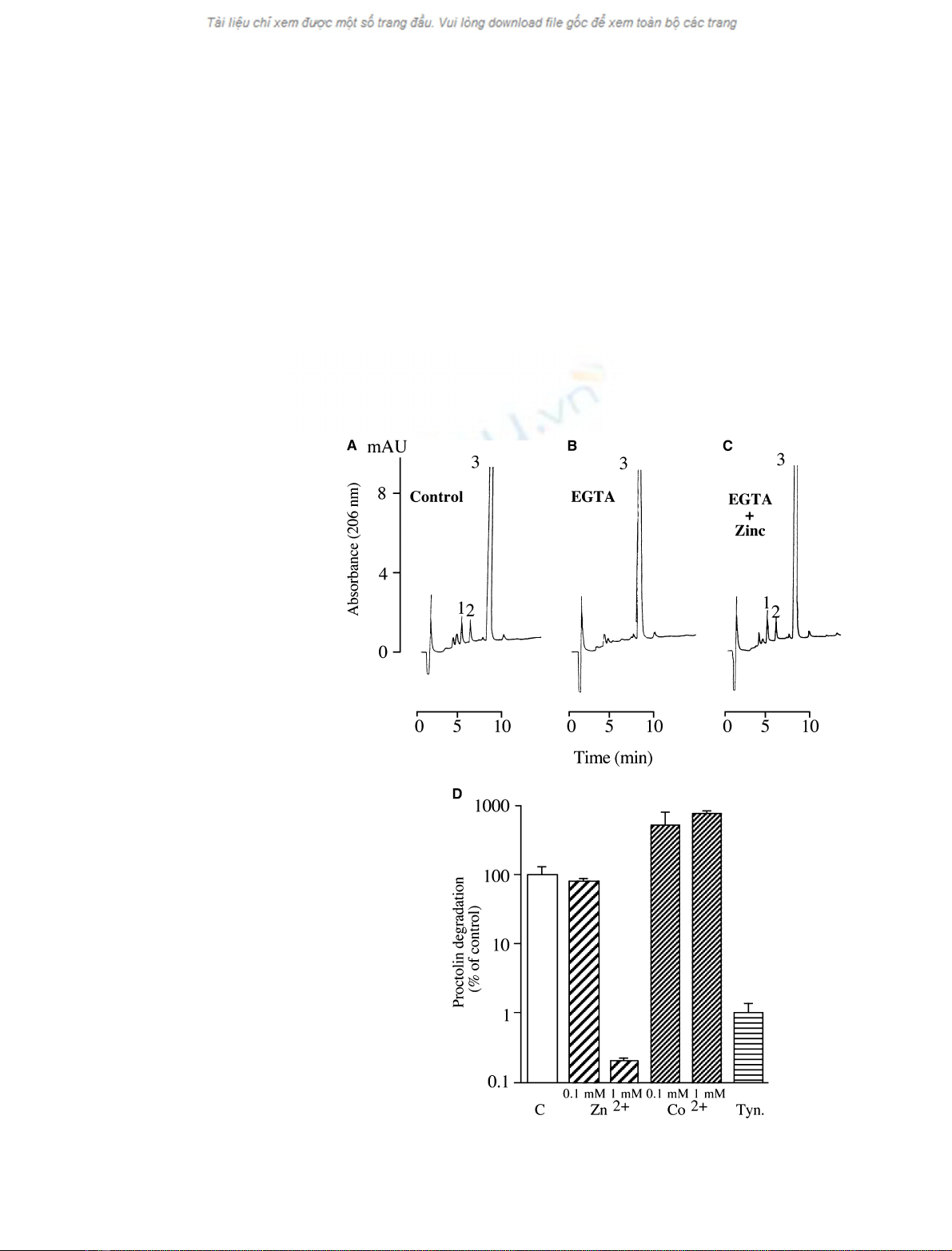
control conditions (Fig. 3A), the addition of the metal
chelator EGTA (1 mm) to the incubation medium pre-
vented 96% of proctolin degradation (Fig. 3B). In
these inhibiting conditions, the addition of 1 mmZn
2+
restored and slightly increased (110%) the proctolin-
degrading activity (Fig. 3C). When the bivalent ion
Zn
2+
was tested at 0.1 mmon the purified enzyme, a
slight inhibition of proctolin-degrading activity (81%
of control) was measured (Fig. 3D). At higher concen-
trations (1 mm), Zn
2+
almost completely prevented
(0.2%) the hydrolysis of proctolin (Fig. 3D). In
Fig. 3. Inhibition and stimulation of the puri-
fied D. melanogaster DPP III. (A–C) Elution
profiles (206 nm) obtained after reversed-
phase separation of samples prepared from
proctolin (250 lM) incubated with purified
D. melanogaster DPP III for 10 min in con-
trol conditions (A), in the presence of 1 mM
EGTA (B) or in the presence of 1 mMEGTA
and 1 mMZnSO
4
(C). The Arg-Tyr dipeptide
(peak 1) and the Leu-Pro-Thr tripeptide (peak
2) were detected after the control incuba-
tions. In contrast, the presence of the metal
chelator in the incubation medium preven-
ted the degradation of proctolin (peak 3),
and no dipeptide or tripeptide could be
detected. When proctolin was incubated
with EGTA and ZnSO
4
, the enzyme activity
was restored and slightly increased (110%
compared with control conditions) as indica-
ted by the detection of both Arg-Tyr (peak 1)
and Leu-Pro-Thr (peak 2). (D) Proctolin
(250 lM) was incubated with purified
D. melanogaster DPP III for 60 min in con-
trol conditions (C), or after preincubation
with 0.1 or 1 mMZnSO
4
(Zn
2+
) or with 0.1
or 1 mMCoCl
2
(Co
2+
) or with the specific
DPP III inhibitor tynorphin (Tyn.) at 10 lM.
The results are mean ± SD from three
experiments.
C. Mazzocco et al.Two DPP III isoforms in the fruit fly
FEBS Journal 273 (2006) 1056–1064 ª2006 The Authors Journal compilation ª2006 FEBS 1059

contrast, the DPP III activity of the purified enzyme
was strongly increased (516% and 758%) by, respect-
ively, 0.1 mmand 1 mmCo
2+
(Fig. 3D). As expected,
the specific DPP III inhibitor tynorphin (10 lm) pre-
vented 99% of proctolin degradation induced by the
purified D. melanogaster DPP III (Fig. 3D).
Central nervous system immunoreactivity
The central nervous system of third-instar larvae of
D. melanogaster was studied with antibody to DPP III.
Cell bodies of nerve cells were positively stained in the
compound ventral ganglion (Fig. 4). The most strongly
immunoreactive cells were visualized at the posterior
end of the ventral ganglion (Fig. 4A). At this location,
most of the cell bodies exhibited significant DPP III
immunoreactivity (Fig. 4B–E), the size of somata ran-
ging from 8 to 10 lm in diameter. Confocal analyses
at high magnification clearly showed that DPP III
immunoreactivity was restricted to the cytosol and
possibly the cell membranes (Fig. 4C). Although the
cell bodies in the ventral ganglion showed strong
DPP III immunoreactivity, stained neurites were scar-
cely observable. Analysis of a series of confocal micro-
scopy slices across the dorsoventral axis of the ventral
ganglion indicated that the somata of stained cells
were uniformly localized at the cortical region of the
ventral ganglion (Fig. 4C–E). When the confocal stud-
ies were focused at the median dorsoventral level, the
segmental neuropil regions were clearly visualized as
bilateral and symmetrical dark masses (Fig. 4C). In
addition, some cortical cell bodies of the posterior
end of the ventral ganglion were visualized by both
Nomarski optic analysis [9] and fluorescence analysis
(Fig. 4F).
Discussion
We here report the characterization of two soluble
DPP III isoforms purified from 40 g fruit flies by three
steps of chromatography. Our results demonstrate that
DPP III is actually expressed in D. melanogaster as
two potently active isoforms and validate both pre-
sumed DPP IIIs annotated from the D. melanogaster
genome [5,6].
In invertebrates, DPP III was first characterized in
Blaberus craniifer as two isoforms of 80 and 76 kDa
[5,10]. Two DPP III-related proteins were also detected
in D. melanogaster at 89 and 82 kDa [5]. Progress in
the sequencing of the D. melanogaster genome allowed
the prediction of first an 82-kDa DPP III [5,6] and
then an 89-kDa DPP III isoform [6]. Both presumed
D. melanogaster DPP III isoforms were functionally
expressed and, indeed, displayed genuine DPP III
activity [7]. We characterized the DPP IIIs actually
detected in D. melanogaster in order to elucidate the
relationship between the 89-kDa and 82-kDa isoforms.
The purified soluble DPP III from D. melanogaster
exhibited similar properties to those of purified B. cra-
niifer DPP III, particularly those of the presumed
D. melanogaster DPP III functionally expressed. When
analysed by electrophoresis and western blotting with
antibody to rat liver DPP III, two major bands at 89
and 82 kDa were detected (Fig. 1A) in the active frac-
tions from size exclusion chromatography. MS ⁄MS
analysis resulted in the sequencing of 60% of both
isoforms, and 100% identity was observed with the
conceptual D. melanogaster DPP IIIs deduced from
the CG7415 gene [6], confirming the results obtained
from the functional expression of the GH01916 clone,
encoding one presumed D. melanogaster DPP III [7].
None of the 45 fragments of D. melanogaster
DPP III examined by MS ⁄MS displayed either phos-
phorylation or glycosylation. The molecular masses
deduced from the 786 and 723 residue theoretical
D. melanogaster DPP IIIs (89 195 and 81 937 Da,
respectively) are closely related to the apparent
molecular masses calculated from SDS ⁄PAGE of the
actual D. melanogaster DPP III proteins (89 and
82 kDa). So, the difference in molecular mass between
the two isoforms, formerly hypothesized to be the
result of post-translational processing of the predicted
82-kDa DPP III protein [5], may be explained by the
sole N-terminal extension of 63 residues retrieved on
the 89-kDa isoform and partly sequenced. In addition,
northern blot analysis of fruit fly total RNAs with a
D. melanogaster DPP III-specific probe revealed two
bands at 2.6 and 2.3 kb (Fig. 1B) that might corres-
pond to the two speculated alternative mRNA tran-
scripts presumably encoding the long or the short
DPP III in this species [6]. Finally, western blot analy-
sis with an anti-rat liver polyclonal antibody never
highlighted a 7-kDa peptide, precluding the 82-kDa
D. melanogaster DPP III resulting from hydrolysis of
the 89-kDa isoform. From these results, it can be con-
cluded that the two D. melanogaster DPP III isoforms
are probably synthesized as two independent native
proteins (89 and 82 kDa), translated from two distinct
alternative mRNAs (2.6 and 2.3 kb) transcribed from
a single gene (CG7415).
The catalytic motif HELLGH-52X-E sequence (all
but three amino acids) was sequenced for both purified
D. melanogaster DPP III isoforms, suggesting that
both are effective in degrading small neuropeptides
such as met-enkephalin and proctolin, although the
89-kDa and 82-kDa isoforms were not assayed sepa-
Two DPP III isoforms in the fruit fly C. Mazzocco et al.
1060 FEBS Journal 273 (2006) 1056–1064 ª2006 The Authors Journal compilation ª2006 FEBS


























