The C-terminal t peptide of acetylcholinesterase forms an ahelix
that supports homomeric and heteromeric interactions
Suzanne Bon
1
, Jean Dufourcq
2
, Jacqueline Leroy
1
, Isabelle Cornut
2
and Jean Massoulie
´
1
1
Laboratoire de Neurobiologie Cellulaire et Mole
´culaire, Ecole Normale Supe
´rieure, Paris, France;
2
Centre de Recherche Paul Pascal,
Pessac, France
Acetylcholinesterase subunits of type T (AChE
T
) possess an
alternatively spliced C-terminal peptide (t peptide) which
endows them with amphiphilic properties, the capacity to
form various homo-oligomers and to associate, as a tetra-
mer, with anchoring proteins containing a proline rich
attachment domain (PRAD). The t peptide contains seven
conserved aromatic residues. By spectroscopic analyses of
the synthetic peptides covering part or all of the t peptide of
Torpedo AChE
T
, we show that the region containing the
aromatic residues adopts an ahelical structure, which is
favored in the presence of lipids and detergent micelles: these
residues therefore form a hydrophobic cluster in a sector of
the helix. We also analyzed the formation of disulfide bonds
between two different AChE
T
subunits, and between
AChE
T
subunits and a PRAD-containing protein [the
N-terminal fragment of the ColQ protein (Q
N
)] possessing
two cysteines upstream or downstream of the PRAD. This
shows that, in the complex formed by four T subunits with
Q
N
(T
4
–Q
N
)
4, the t peptides are not folded on themselves as
hairpins but instead are all oriented in the same direction,
antiparallel to that of the PRAD
5. The formation of disulfide
bonds between various pairs of cysteines, introduced by
mutagenesis at various positions in the t peptides, indicates
that this complex possesses a surprising flexibility.
Keywords: acetylcholinesterase; amphiphilic alpha helix;
disulfide bonds; proline rich domain.
The quaternary associations of acetylcholinesterase (AChE)
and butyrylcholinesterase (BChE) are determined by small
C-terminal domains that are distinct from the catalytic
domain [1,2]. In vertebrates, alternatively spliced exons of
the AChE gene
6encode several C-terminal domains which
distinguish different types of subunits. However, only
subunits of type T (tailed) exist in the BChE and AChEs
of all vertebrates; in mammals they represent the only
AChE variant expressed in the adult nervous system and
muscles. These subunits possess specific association pro-
perties, which depend on their C-terminal t peptide. This
peptide is strongly conserved in vertebrates, with 75%
identity between cartilagenous fishes (Torpedo) and mam-
mals; it contains 40 or 41 residues, with a cysteine at )4from
the C-terminus and a series of seven conserved aromatic
residues including three tryptophans [3].
Transfected COS cells expressing subunits of type T
produce a wide array of catalytically active AChE forms,
including monomers, dimers and tetramers [4]. The mono-
mers, dimers and some tetramers are amphiphilic, as defined
by their interaction with detergent micelles, which modify
their sedimentation and their electrophoretic migration in
nondenaturing conditions [5]. These amphiphilic molecular
forms require detergents to be totally solubilized but are also
secreted when expressed in transfected COS cells [4]. The
t peptide is necessary for the amphiphilic character of
AChE and for the formation of tetramers, as deleted
subunits that lack this peptide generate only nonamphiphilic
monomers [6].
AChE subunits of type T (AChE
T
) can assemble into
tetramers with their anchoring proteins ColQ and PRiMA,
and these heteromeric associations represent the physio-
logically functional species in muscles and brain [7,8]. At the
neuromuscular junction, collagen-tailed asymmetric forms
are inserted in the basal lamina; in these molecules, one
AChE
T
tetramer (T
4
) is attached to the N-terminal region of
each of the three strands of the triple helical ColQ collagen.
In the mammalian brain, the predominant AChE species
is a tetramer, anchored at the cell surface through the
Correspondence to S. Bon, Laboratoire de Neurobiologie Cellulaire
et Mole
´culaire, CNRS UMR 8544, Ecole Normale Supe
´rieure,
46 rue d’Ulm, 75005 Paris, France.
Fax: + 33 1 44 32 38 87, Tel.: + 33 1 44 32 38 91,
E-mail: jean.massoulie@biologie.ens.fr
Abbreviations: AChE, acetylcholinesterase; AChE
H
, AChE subunit of
type H; AChE
T
, AChE subunit of type T (tailed); BChE, butyryl-
cholinesterase; BChE
T
, BChE subunit of type T (tailed); cmc, critical
micellar concentration; CTAB, cetyltrimethylammonium bromide;
C37, C-terminal cysteine residue at position 37; GPI, glycophospha-
tidylinositol; PI-PLC, phosphatidylinositol-specific phospholipase C;
PRAD, proline rich attachment domain; Q
N
, N-terminal fragment of
the ColQ protein; SMCC, N-succinimidyl-4-(N-maleimidomethyl)
cyclohexane-1 carboxylate; t peptide, the C-terminal peptide of
AChE
T
subunits; T, AChE
T
subunits; WAT, tryptophan amphiphilic
tetramerization domain.
Note: In this paper the residues of the t peptides of AChE
T
from
different species are numbered from 1 to 40 in order to facilitate
comparisons.
(Received 31 July 2003, revised 10 October 2003,
accepted 23 October 2003)
Eur. J. Biochem. 271, 33–47 (2004) FEBS 2003 doi:10.1046/j.1432-1033.2003.03892.x

transmembrane protein PRiMA (T
4
–PRiMA). The
N-terminal regions of both ColQ and PRiMA contain a
proline-rich attachment domain (PRAD) [9], which is
responsible for their interaction with AChE
T
or BChE
T
subunits; in addition, they contain cysteines that form
disulfide bonds with two cholinesterase T subunits in each
tetramer, by means of the cysteines located near their
C-terminus [10–12].
The t peptide is in fact sufficient for association with a
PRAD, as shown by the fact that it can replace a complete
AChE
T
or BChE
T
subunit in PRAD-associated tetramers,
and can induce the formation of PRAD-linked tetramers
when added at the C-terminus of foreign proteins such as
green fluorescent protein or alkaline phosphatase: it there-
fore constitutes an autonomous interaction domain,
referred to as the WAT [tryptophan amphiphilic tetra-
merization] domain [13]. The t peptide also acts as an
enhancer of degradation through the ER-associated degra-
dation pathway [14].
In the present study, we analyse the structural basis for
the hydrophobic and quaternary interactions of the t pep-
tide. In particular, we ask whether hydrophobic interactions
result from the structure of the peptide itself or require post-
translational modifications, e.g. the addition of lipidic
residues. It has been reported that membrane-bound mouse
AChE produced in transfected human embryo kidney 293
cells incorporates palmitic acid, but not mevalonate, in spite
of the resemblance of its C-terminus with an isoprenylaytion
signal [15].
The amphiphilic properties of AChE
T
subunits suggest
that the t peptide constitutes an amphiphilic ahelix, with its
seven aromatic residues located in the same sector, forming
a hydrophobic cluster [1]. Here, we present evidence that the
t peptide actually forms an amphiphilic helix and that it is
elongated, rather than folded upon itself in a hairpin as
proposed by Giles [16], in AChE
T
monomers and dimers as
well as in tetramers associated with an N-terminal fragment
of ColQ (Q
N
). We also show that the four t peptides are
parallel to each other and antiparallel
7to the PRAD in the
T
4
–Q
N
heteromeric complex.
Materials and methods
Materials
Egg phosphatidylcholine and its lyso derivative were
prepared as described previously [17]. Phosphatidylserine
was obtained from Lipid Products (Nutfield, Surrey, UK).
The detergents used for the spectroscopic studies were from
VWR (Strasbourg, France) and Sigma
8and were recrystal-
lized before use. A lytic tetrameric form (G
4
) derived from
collagen-tailed Electrophorus AChE was purified by affinity
chromatography on Sepharose derivatized with hexylamido-
carboxyphenyl-dimethylethylammonium, as described pre-
viously [18].
Peptide synthesis
The t
1)32
peptide was synthesized in the laboratory of
J. Vandekerckhove (Laboratorium Genetika, Gent, Bel-
gium). It was purified by preparative HPLC and analyzed in
a C-18 Vydac column (The Nest Group, Southborough,
MA, USA): the preparation contained essentially only the
monomeric peptide, with less than 10% dimers, spontane-
ously formed upon air oxidation and that could be reduced
by dithiothreitol. The t
1)40
peptide, at 85% purity, was
synthesized by Neosystem Laboratoires (Strasbourg,
France). The t
25)40
peptide was synthesized in the laboratory
of J. Igolen (Institut Pasteur, Paris, France) and was puri-
fied by preparative HPLC. Whereas the C-terminal cysteine
residue at position 37 of t
1)40
(C37) was blocked by an
acetamidomethyl group, cysteines were added at the N-ter-
minus of t
1)32
and t
1)40
, to allow their linkage to non-
amphiphilic AChE tetramers from Electrophorus electric
organs, via their N-terminal extremity, as with AChE
T
subunits.
Chemical coupling of peptides with
Electrophorus
G
4
AChE
Each of the t
1)32
,t
1)40
and t
25)40
peptides were covalently
coupled to the G
4
form of Electrophorus AChE by the
heterobifunctional reagent N-succinimidyl-4-(N-maleimido-
methyl)cyclohexane-1 carboxylate (SMCC). This method
involves the reaction of thiol groups from cysteine residues
of the peptides with a maleimido group incorporated into
AChE after reaction with SMCC. The preparation of
AChE–SMCC has been described elsewhere [19].
Subsequently to being dissolved in 0.1
M
phosphate
buffer, pH 6, the thiol content of the peptides was measured
by reaction with 5,5¢-dithiobis(2-nitrobenzoic acid) [20].
Coupling between the peptide and the enzyme was obtained
by mixing AChE–SMCC with an excess of thiol groups (the
concentration of thiol was 100-fold that of G
4
). Peptides
t
1)32
and t
1)40
were coupled using the added N-terminal
cysteine and t
25)40
was coupled through C37. After 3 h at
30 C, the conjugate was purified by molecular sieve
chromatography in a Biogel A0.5 column (Bio-Rad
Laboratories), as described previously [21]. We observed
no significant loss in enzyme activity during the coupling
procedure.
Production of antibodies against t
25)40
peptide
Anti-(t
25)40
) polyclonal Ig was raised in rabbit against the
t
25)40
peptide covalently coupled to BSA. The t
25)40
–BSA
conjugate was obtained by reaction with glutaraldehyde, as
described previously [22]. Immunization followed the pro-
cedure described by Vaitukatis [23].
Spectroscopic analyses
Circular dichroism spectra were obtained in an AVIV 62DS
(AVIV, Zu
¨rich, Switzerland) spectrometer at 25 C, using
cuvettes of 0.1–1 cm path-length according to the concen-
tration of peptide. The blank was subtracted in all cases. For
evaluation of the molar ellipticity per residue (h) expressed
in degÆdmol
)1
Æcm
2
, the peptide concentration was calculated
by using an absorbance e
280
¼20 000
M
–l
Æcm
–l
.
Fluorescence spectra were obtained with a Fluoromax
SPEX spectrophotometer (Jobin et Yvon, Longjumeau,
France)at25C, with an excitation wavelength of 280 nm
and a slit width of 1.7 nm. The spectra corresponding to an
average of at least two or three scans were corrected in
34 S. Bon et al. (Eur. J. Biochem. 271)FEBS 2003

emission, and the background fluorescence from buffer and
detergent were subtracted.
Mutagenesis and transfections
cDNA encoding rat AChE subunits was inserted in the
pEF-BOS vector, which is under the control of the human
EF-10c promotor; this vector was used for mutagenesis and
expression in COS cells [4]. All constructs were identical,
except for the 3¢sequence encoding the C-terminal peptides.
AChE
T
subunits were coexpressed with proteins derived
from Q
N
, containing either the natural PRAD motif with
its two adjacent cysteines upstream of the proline-rich
segment (CC-Q
N
), or a modified PRAD, in which these
cysteines were replaced by serines, and two cysteines were
introduced downstream of the prolines (Q
N
-CC). A Q
N
construct from which the PRAD was deleted (residues 70–
86) was used in control cultures, to ensure an identical level
of AChE
T
expression. In a number of experiments we used
a construct that contained a C-terminal GPI addition signal
derived from Torpedo type H AChE (AChE
H
) subunits, so
that the resulting complex, (AChE
T
)
4
–Q
N
–GPI, could be
recovered from the cell surface by treatment with phos-
phatidylinositol-specific phospholipase C (PI-PLC). For
transfections, DNA was purified on Nucleobond AX
columns (Macherey–Nagel, Hoerdt, France). COS-7 cells
were transfected by the diethylaminoethyl-dextran method,
as described previously [9]. The cells were maintained at
37 C and were collected after three days.
Preparation of extracts and AChE assay
The cells were extracted with TMg buffer [1% (v/v) Triton
X-100; 20 m
M
Tris/HCl pH 7.5; 10 m
M
MgCl
2
]at4C
when the AChE
T
subunits were expressed alone or with Q
N
,
and at 20 C when they were expressed with a Q
N
–GPI
construct, because the GPI-anchored complex is associated
with sphingolipid/cholesterol microdomains which remain
partially insoluble in Triton X-100 in the cold.
The AChE activity was assayed by the colorimetric
method of Ellman [20]. Enzyme samples (10 lL) were
added to 0.2 mL of Ellman assay medium and the reaction
kinetics were monitored at 414 nm, at 15 s intervals over a
3 min period, using a Multiskan RC microplate reader
(Labsystems, Helsinki, Finland).
Sucrose gradients and nondenaturing electrophoresis
Aliquots of extracts (typically 200 lL) containing 1% (v/v)
Brij-96 buffer (10 m
M
MgCl
2
,25m
M
Tris/HCl pH 7) were
loaded on 5–20% (w/v) sucrose gradients in 1% (v/v) Brij-
96 buffer. Escherichia coli b-galactosidase (16 S) and
alkaline phosphatase (6.1 S) were included as internal
sedimentation standards. The gradients were centrifuged
for 18 h at 36 000 r.p.m. at 5 C, in a LE80K centrifuge
using an SW-41 rotor (Beckman–Coulter, Villepinte,
France). Fractions of 300 lL were collected and assayed
for AChE, b-galactosidase and alkaline phosphatase
activities. Electrophoresis in nondenaturating polyacryl-
amide gels was performed as described previously [24] and
AChE activity was shown by the histochemical method of
Karnovsky and Roots [25].
Metabolic labeling
Two days after cotransfection of AChE
T
subunits with the
Torpedo AChE
H
C-terminal addition signal, the transfected
COS cells were preincubated for 45 min in Dulbecco’s
modified Eagle’s medium lacking cysteine and methionine,
and then labeled with [
35
S]methionine–cysteine (Amersham
Biosciences) for 3 h. The cells were then rinsed with NaCl/
P
i
, and chased overnight in a medium containing Nu-serum
(BD Biosciences, Bedford, MA, USA). The cell surface
GPI-anchored AChE was solubilized by treating intact cells
for 2 h at 37 C with PI-PLC (1 : 600) from Bacillus
thuringiensis, kindly provided by I. Silman (Weizmann
Institute, Rehovot, Israel). Following centrifugation at
10 000 gfor 15 min to remove cell debris, the soluble
enzyme (secreted and PI-PLC released) was collected for
immunoprecipitation.
Immunoprecipitation and SDS/PAGE
AChE from cell extracts or medium were immunoadsorbed
on protein G immobilized on Sepharose 4B Fast Flow
beads (Sigma). The beads were first washed and saturated
with 5% (v/v) BSA in a buffer containing 150 m
M
NaCl,
5m
M
EDTA, 50 m
M
Tris/HCl pH 7.4, 0.05% (v/v) NP40.
Samples of 1.5 mL of cell extracts or media were incubated
with 40 lL of a 10% suspension of beads for 3 h to
eliminate nonspecific adsorption and the beads were
discarded. The samples were incubated with 1 : 500 anti-
(rat AChE) serum A63 [26] overnight at 8 C, with gentle
agitation on a rotating wheel, followed by addition of 80 lL
of a 10% suspension of BSA-saturated washed beads and
incubation for 1 h. After immunoadsorbtion, the beads
were washed and centrifuged three times with 1 mL of
buffer containing 1% Triton X-100 and centrifugations at
10 000 gfor 5 min. All incubations were performed at 8 C
under mild rotational agitation.
For polyacrylamide electrophoresis under denaturing
conditions, samples of the washed beads were resuspended
in 30 lLof0.125
M
Tris/HCl buffer pH 6.8 containing 1%
SDS, 0.002% bromophenol blue, 5% 2-mercaptoethanol
(v/v/v), heated at 98 C for 5 min, and centrifuged at
10 000 gfor 5 min at room temperature. Aliquots of 10 lL
of the supernatant were submitted to electrophoresis in
SDS/polyacrylamide gels, and the resulting bands were
revealed with the BAS 1000 Fuji Image analyzer (Fujifilm,
St Quentin-en-Yvelines, France) or by autoradiography,
and analyzed with the Fuji Image
GAUGE
software.
Prediction of secondary structure elements
The secondary structure of the C-terminal region of the
catalytic domain and of the t peptide was predicted
according to Rost [27] using
PREDICTPROTEIN
at http://
maple.bioc.columbia.edu/predictprotein.
Results
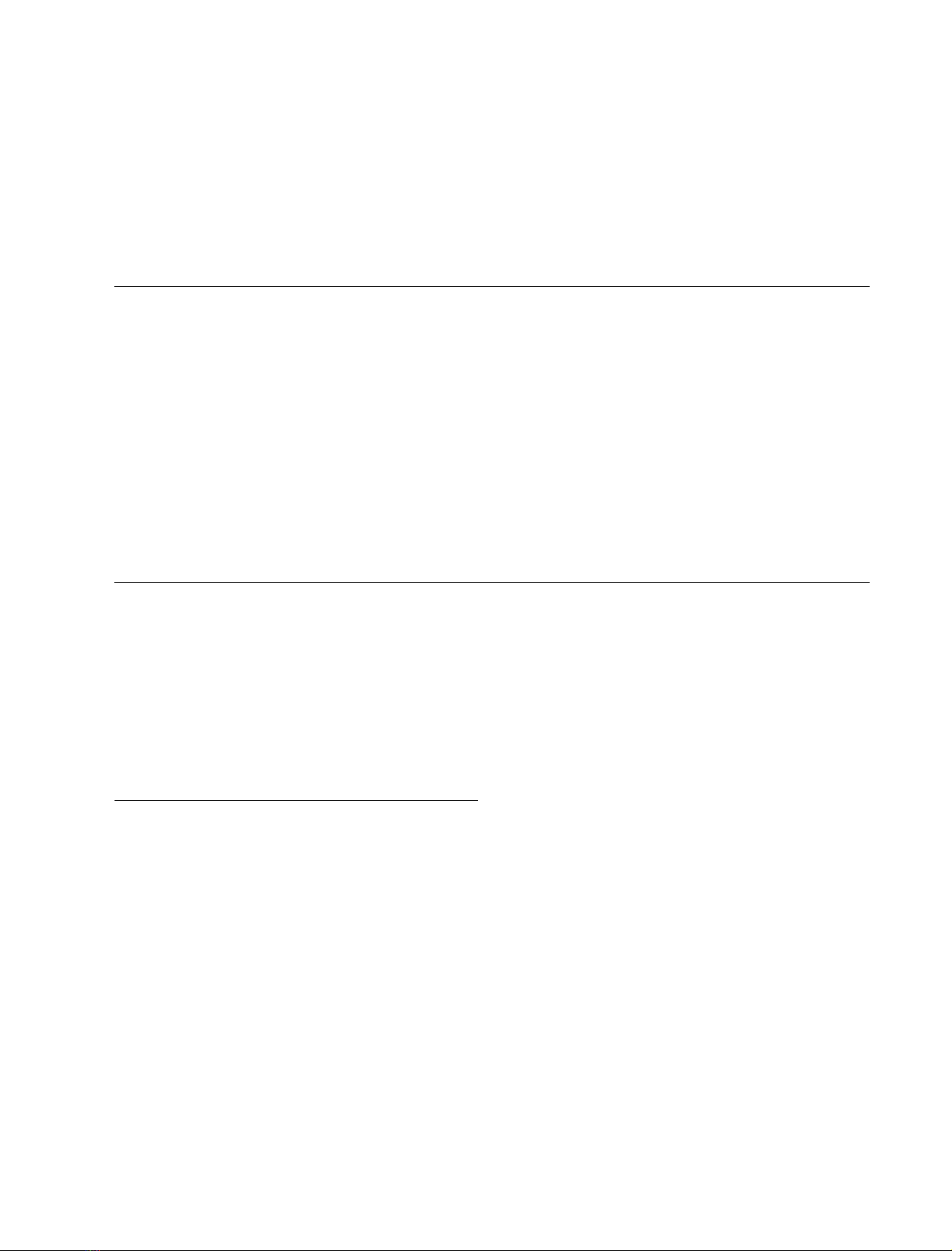
Modeling of the t peptide as an amphiphilic ahelix
The primary sequence of the C-terminal region of Torpedo
AChE
T
is shown in Fig. 1A, including the last 12 residues of
FEBS 2003 Amphiphilic ahelical domain of the AChE T subunit (Eur. J. Biochem. 271)35
the catalytic domain and the t peptide. Secondary structure
prediction algorithms show that a large part of this peptide
is expected to assume an ahelical structure, extending from
residue five to residue 26 or 28, with a possible interruption
at residues 14–16 that might allow a bend between two
helical segments. Giles proposed a similar arrangement, in
which a bend at residues 21–22 would bring together the
aromatic sectors of the two helices [16]; according to this
model, residues located in the N-terminal region of the
t peptide would be in close contact with the C-terminal
cysteine, C37.
If we assume an ahelical structure for the t peptide, a
lateral view shows that all the aromatic residues are oriented
on the same side (Fig. 1B), and a wheel projection [28] shows
that a sector of 100is totally apolar (Fig. 1B). The polar
sector contains five acidic residues (one aspartic and four
glutamic acids) and four basic residues (one lysine, two
arginines and one histidine), which might form internal salt
bridges between residues D4 or E5 and R8, between E7 and
K11, and between E13 and R16, as analyzed in a further
study (S. Belbeoc’h, J. Leroy, A. Ayon, J. Massoulie
´&
S. Bon, unpublished results). The cluster of hydrophobic side
chains in the apolar sector includes the seven aromatic
residues that are conserved in all known vertebrate AChEs
and BChEs, ranging from cartilagenous fishes (Torpedo)to
mammals. In particular, three tryptophans are evenly spaced
by seven residues and very close to each other in the wheel
diagram (Fig. 1B). This aromatic cluster could be respon-
sible for the hydrophobic interactions of AChE
T
subunits.
Chemical grafting of synthetic peptides confers
hydrophobic properties on water-soluble AChE
To characterize the interactions of the t region while
excluding possible effects of putative post-translational
modifications, we used chemically synthesized peptides, as
shown in Fig. 1C. Peptide t
1)40
corresponds to the whole
Torpedo t peptide; peptide t
1)32
corresponds to its first 32
aminoacids and contains all seven conserved aromatic
residues.
The peptides were grafted onto a water-soluble tetrameric
form (G
4
)ofElectrophorus electricus AChE, obtained by
tryptic digestion of collagen-tailed forms from the electric
organ [29,30]. We used this enzyme preparation because we
could obtain it in a highly purified form [18] and because
it was very stable, totally nonamphiphilic and could be
Fig. 1. Sequence and putative organization of
the C-terminal t peptide from AChE
T
.
(A) Primary structure of the last 12 residues of
the catalytic domain and of the t peptide.
A comparison of the Torpedo and rat
sequences shows the high degree of conserva-
tion, particularly of the seven aromatic resi-
dues, throughout vertebrates. The N-terminal
region of the human amyloid Abpeptide is
shown to indicate a 12 residue segment which
presents some homology with the t peptide
(underlined) (B) Proposed helical structure of
the N-terminal region of the t peptide: in the
side view, the distance of each residue from the
helix axis corresponds to the vertical dimen-
sion, with the central residue of the aromatic
cluster (W17) at the top. The position along
the axis corresponds to the horizontal dimen-
sion (arbitrary scales). The wheel representa-
tion corresponds to a faceview along the helix
axis of the segment of the t peptide containing
the aromatic residues. (C) Synthetic peptides
corresponding to different parts of the
t peptide. The underlined residues have been
substituted from the wildtype sequence of the
Torpedo marmorata tpeptide.
36 S. Bon et al. (Eur. J. Biochem. 271)FEBS 2003
analyzed by the same methods used for the amphiphilic
AChE species. Chemical coupling of the synthetic peptides
to exposed lysine residues occurred randomly and did not
affect enzymic activity.
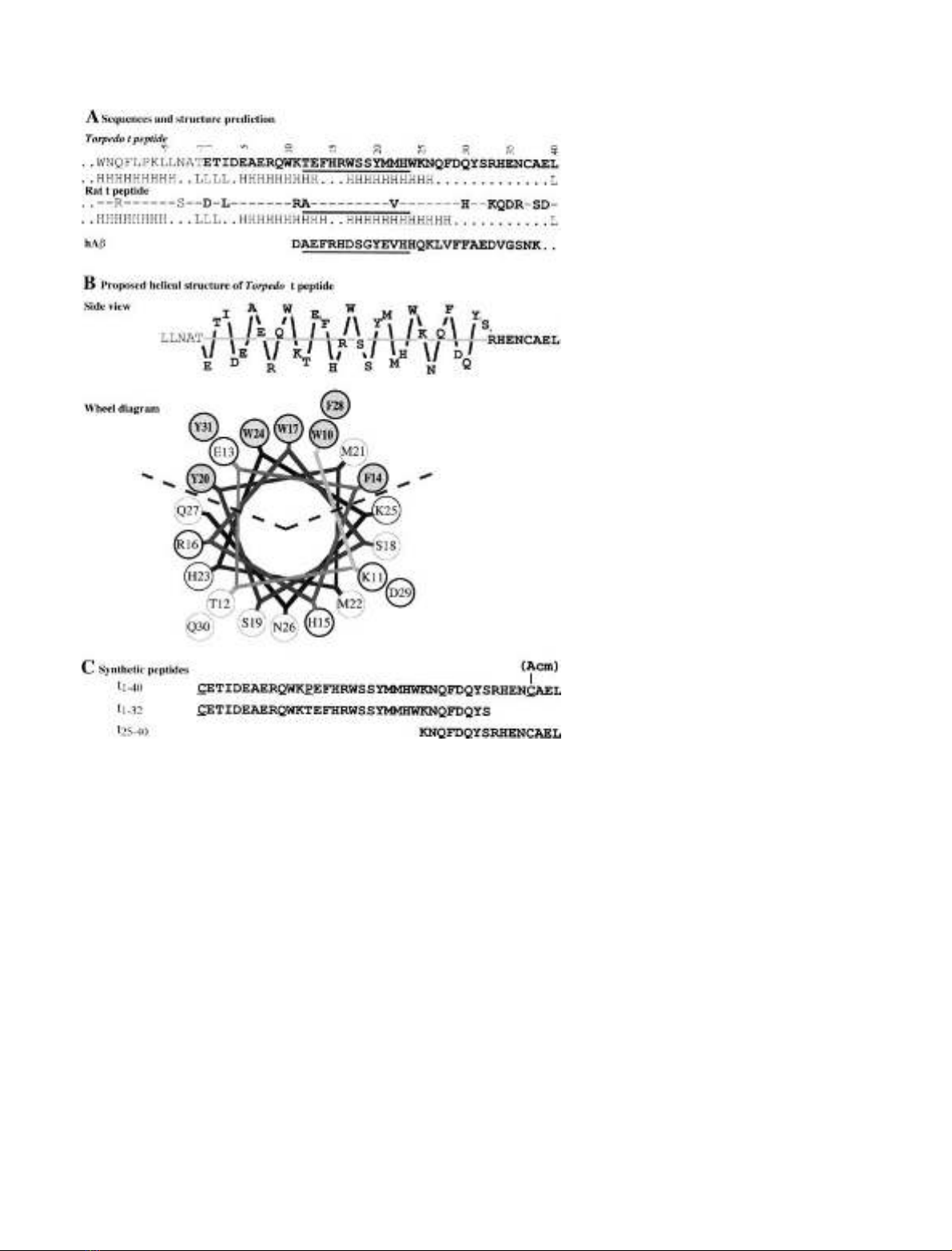
We deduced the mean number of peptides added per
tetramer from the apparent increase in molecular mass: the
modified Electrophorus G
4
AChE molecules obtained after
coupling of the peptides sedimented as fairly homogenous
peaks, as illustrated in Fig. 2A. The sedimentation coeffi-
cient of G
4
-t
1)32
and of G
4
-t
1)40
was about 12.8 S, as
compared to 11.8 S for the original G
4
form (Fig. 2B).
Assuming that the mass of this globular protein is propor-
tional to S
3/2
, we estimate that the mass of the tetramer
increased from 320 kDa to 360 kDa, i.e. 10 kDa per
subunit, which corresponds to an average of three grafted
peptides per AChE subunit. In the case of G
4
-t
1)40
and
G
4
-t
25)40
, the formation of complexes with antibodies raised
against t
25)40
confirmed that essentially all the Electrophorus
G
4
AChE molecules had been modified (not shown). The
G
4
-t
1)32
derivative did not bind the antibodies, indicating
that the t
1)32
peptide did not contain the necessary epitopes.
The G
4
-t
25)40
derivative, like the original Electrophorus
G
4
enzyme, was not amphiphilic: its sedimentation coeffi-
cient (12.9 S) was not influenced by the presence of
detergent in the gradients. By contrast, the G
4
-t
1)32
and
G
4
-t
1)40
derivatives were clearly amphiphilic, as they
sedimented more slowly in the presence of Triton X-100
and even more slowly in the presence of Brij-96 (Fig. 2A,B).
This amphiphilic character was confirmed by charge-shift
electrophoresis under nondenaturing conditions. The
t-peptide–AChE conjugates migrated in opposite directions
in the presence of the negatively and positively charged
detergents, cetyltrimethylammonium bromide (CTAB) and
Na
+
deoxycholate (not shown).
The fact that the short t
1)32
peptide and the long t
1)40
peptide confer amphiphilic properties to Electrophorus
AChE tetramers, whereas the t
25)40
peptide does not
suggests that the 1–32 region, containing an ahelix with
seven aromatic residues, is sufficient to support hydropho-
bic interactions.
Characterization of t peptide–lipid interactions
by use of circular dichroism
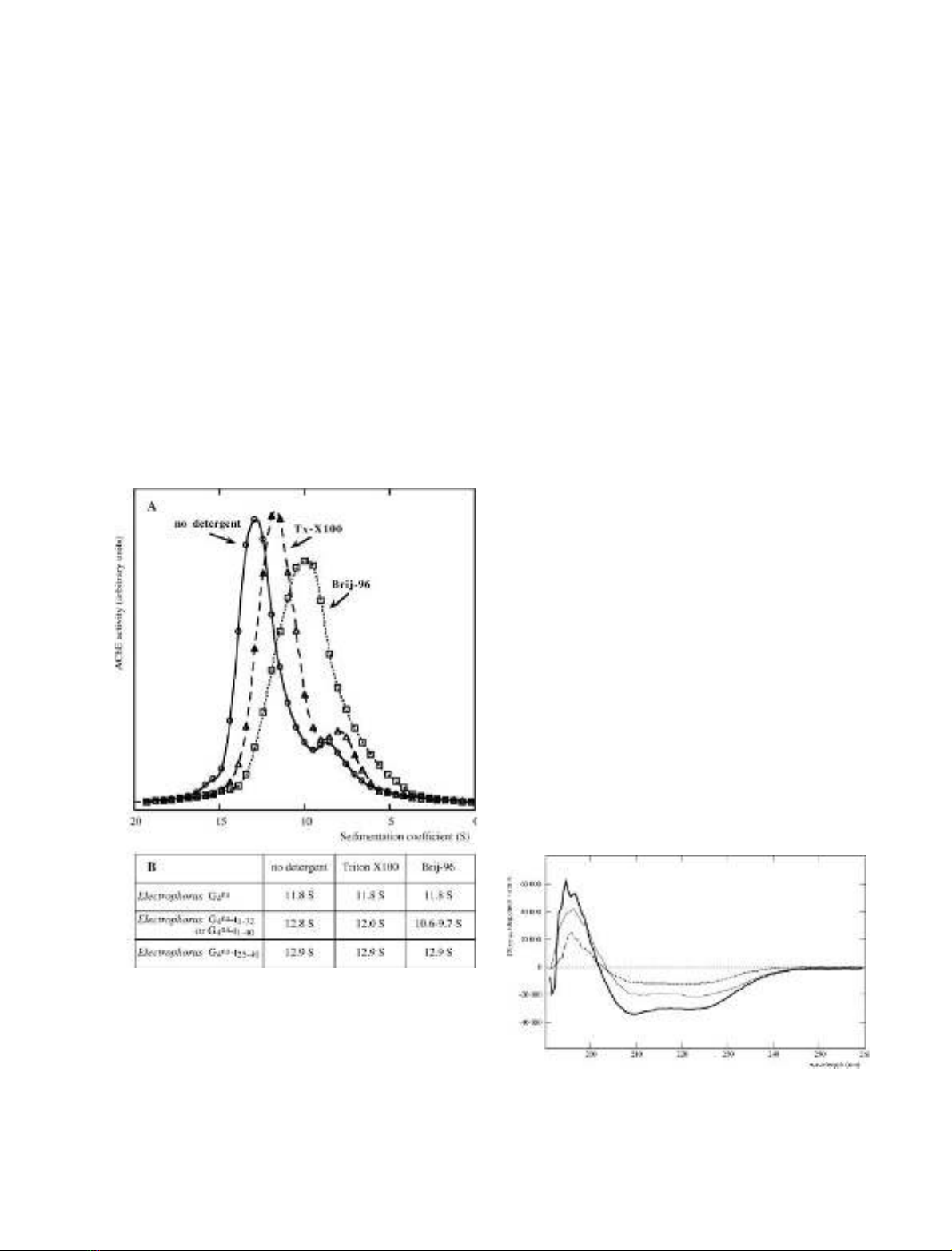
Figure 3 shows the CD spectrum in the far UV of the t
1)32
peptide under various conditions. In organic solvents, such
as methanol, the spectrum presents the characteristic
features of an ahelical structure, with double minima at
210 nm and 222 nm. The h
222
value of )31 600 degÆdmol
)1
Æ
cm
2
indicates that about 85% of the polypeptide is ahelical.
We obtained a similar proportion of ahelical structure by
reconstituting the whole spectrum as a sum of the contri-
butions of different secondary structures, derived from a
set of known proteins [31]. This high ahelical content
is comparable to that of amphiphilic peptides of similar
length, which have been characterized by various methods
as monomeric 20-residue ahelical rods [32,33]. When the
peptide was dissolved in an aqueous buffer, the minima at
210 nm and 222 nm displayed ellipticities of only
h¼)12 210 degÆdmol
)1
Æcm
2
and h¼)9770 degÆdmol
–l
Æcm
2
respectively, indicating a much lower ahelical content of
35%.
Fig. 2. Effect of detergents on the sedimentation of Electrophorus AChE
tetramers, chemically coupled with the t
1)40
peptide. (A) Sedimentation
patterns of a conjugate of Electrophorus AChE G
4
species with the
t
1)40
peptide, obtained in sucrose gradients containing no detergent;
0.1% Triton X-100 or 0.1% Brij-96. (B) Sedimentation coefficients
obtained in these different conditions for G
4
AChE and its conjugates.
The conjugated enzymes containing peptides t
1)32
and t
1)40
sedi-
mented faster without detergent than in the presence of Triton X-100
or Brij-96, indicating that they bind detergent micelles, in contrast with
conjugated enzyme containing peptide t
25)40
and the nonconjugated
enzyme, which sedimented in the same way under all three conditions.
Fig. 3. Far UV dichroic spectrum of peptide t
1-32
.Peptide (5 l
M
)in
1m
M
Tris/HCl buffer, pH 7.5, using a 1 cm path-length cuvette
(dotted line); the same solution after addition of lysolecithin micelles,
with a lipid/peptide molar ratio of 20 (thin line); 50 l
M
peptide in
methanol, using a 0.1 path-length cuvette (bold line).
FEBS 2003 Amphiphilic ahelical domain of the AChE T subunit (Eur. J. Biochem. 271)37


























