Effect of ibuprofen and warfarin on the allosteric properties of
haem–human serum albumin
A spectroscopic study
Simona Baroni
1
, Marco Mattu
2
, Alessandro Vannini
2
, Rita Cipollone
2
, Silvio Aime
1
, Paolo Ascenzi
2
and
Mauro Fasano
3
1
Department of Chemistry ‘IFM’, University of Torino, Italy;
2
Department of Biology, University ‘Roma Tre’, Rome, Italy;
3
Department of
Structural and Functional Biology, University of Insubria, Italy
Haem binding to human serum albumin (HSA) endows the
protein with peculiar spectroscopic properties. Here, the
effect of ibuprofen and warfarin on the spectroscopic
properties of ferric haem–human serum albumin (ferric
HSA–haem) and of ferrous nitrosylated haem–human
serum albumin (ferrous HSA–haem-NO) is reported. Ferric
HSA–haem is hexa-coordinated, the haem-iron atom being
bonded to His105 and Tyr148. Upon drug binding to the
warfarin primary site, the displacement of water molecu-
les 2buried in close proximity to the haem binding
pocket 2induces perturbation of the electronic absorbance
properties of the chromophore without affecting the
coordination number or the spin state of the haem-iron,
and the quenching of the
1
H-NMR relaxivity. Values of
K
d
for ibuprofen and warfarin binding to the warfarin
primary site of ferric HSA–haem, corresponding to the
ibuprofen secondary cleft, are 5.4 ^1.1 10
24
Mand
2.1 ^0.4 10
25
M, respectively. The affinity of ibuprofen
and warfarin for the warfarin primary cleft of ferric HSA–
haem is lower than that reported for drug binding to haem-
free HSA. Accordingly, the K
d
value for haem binding to
HSA increases from 1.3 ^0.2 10
28
Min the absence of
drugs to 1.5 ^0.2 10
27
Min the presence of ibuprofen
and warfarin. Ferrous HSA–haem-NO is a five-coordinated
haem-iron system. Drug binding to the warfarin primary site
of ferrous HSA–haem-NO induces the transition towards
the six-coordinated haem-iron species, the haem-iron atom
being bonded to His105. Remarkably, the ibuprofen primary
cleft appears to be functionally and spectroscopically
uncoupled from the haem site of HSA. Present results
represent a clear-cut evidence for the drug-induced shift of
allosteric equilibrium(a) of HSA.
Keywords: allostery; haem–human serum albumin; human
serum albumin; ibuprofen; warfarin.
Human serum albumin (HSA), the most prominent protein
in plasma, is best known for its exceptional ligand binding
capacity, the most strongly bound compounds being
hydrophobic organic anions of medium size, long-chain
fatty acids, haem and bilirubin. Smaller and less
hydrophobic compounds (e.g. tryptophan) are held less
strongly, but their binding can still be highly specific. For
many compounds, HSA provides a depot so they will be
available in quantities well beyond their solubility in
plasma. Moreover, HSA abundance (concentration of
45 mg:mL
21
in the serum of human adults) makes it an
important determinant of the pharmacokinetic behaviour of
many drugs. In other cases, HSA holds some ligands in a
strained orientation, allowing their metabolic modification,
and renders potential toxins harmless by transporting them
to disposal sites. HSA also accounts for most of the
antioxidant capacity of human serum, either directly or by
binding and carrying radical scavengers, or by sequestering
transition metal ions with pro-oxidant activity. Finally, HSA
acts as a nitric oxide depot and carrier, leading to covalent
modification(s) of (macro)molecules [1–8].
The amino acid sequence of HSA shows the occurrence of
three homologous domains, probably arising from divergent
evolution of a degenerated ancestral gene followed by a
fusion event. However, the three domains deduced from the
primary structure do not correspond to domains found in
the three-dimensional model. Rather, terminal regions of
sequential domains contribute to the formation of inter-
domain helices linking domain I to II, and II to III,
respectively. On the other hand, each domain is known to
consist of two separate sub-domains, connected by a random
coil. Therefore, HSA can be considered as an ensemble of
four globular domains, namely IA, IB 1IIA, IIB 1IIIA,
and IIIB, freely linked by extended random coils. It is thus
reasonable to hypothesize allosteric conformational tran-
sition(s) occurring in HSA upon ligand binding. Note that
the flexibility of the HSA structure allows it to adapt
readily to ligands and that its three-domain design provides
a variety of binding sites. In particular, the conformational
adaptability of HSA involves more than the immediate
Correspondence to M. Fasano, Department of Structural and Functional
Biology, University of Insubria, Via Jean H. Dunant 3, I-21100 Varese,
Italy. Fax: 139 0332 421500, Tel. : 139 0332 421523,
E-mail: mauro.fasano@uninsubria.it
Note: S. Baroni and M. Mattu contributed equally to this work.
(Received 2 July 2001, revised 27 September 2001, accepted 3 October
2001)
Abbreviations: HSA, human serum albumin; ferric HSA–haem, ferric
haem–human serum albumin; ferrous HSA–haem-NO, ferrous
nitrosylated haem–human serum albumin.
Eur. J. Biochem. 268, 6214–6220 (2001) qFEBS 2001
vicinity of the binding site(s), affecting both the structure
and the ligand binding properties of the whole HSA
molecule [1–11].
The interaction of ligands with HSA occurs mainly in two
regions. According to the Sudlow’s nomenclature, bulky
heterocyclic anions bind to site I (located in subdomain IIA),
whereas site II (located in subdomain IIIA) is preferred by
aromatic carboxylates with an extended conformation.
Remarkably, ibuprofen, a nonsteroidal anti-inflammatory
agent [12], and warfarin, an anticoagulant drug [12], are
considered as stereotypical ligands for Sudlow’s site II and
Sudlow’s site I, respectively [1,9,11,13,14]. Ibuprofen binds
to Sudlow’s site II with K
d
¼3.7 10
27
M[1,15], whereas
warfarin binds to Sudlow’s site I with K
d
¼3.0 10
26
M
[1,16–18]. Secondary binding clefts have been found for
ibuprofen and warfarin to be located on domain I.
Remarkably, the ibuprofen secondary site corresponds to
the warfarin primary cleft (i.e. to Sudlow’s site I) [1,3,11].
Moreover, multiple recognition sites for binding of
anaesthetics, fatty acids, and triiodobenzoic acid to HSA
have also been identified [2,5,6,14,19].
Among hydrophobic molecules, haem binding to HSA is
of peculiar relevance for the haem iron reuptake following
hemolytic events [1,20]. The haem binding site has been
located primarily at the interface between domains I and II
of HSA, while on domains II and III secondary binding
clefts have been found [3,7,10]. The binding of this
spectroscopically active label to HSA makes it possible to
follow a number of events involving the holoprotein by
taking advantage of electronic absorption spectroscopy,
EPR spectroscopy and
1
H-NMR relaxometry [7,8,10,21,22].
Here, the effect of ibuprofen and warfarin on the electronic
absorption spectroscopic and
1
H-NMR-relaxometric proper-
ties of ferric haem – human serum albumin (ferric HSA–
haem) as well as on the EPR spectroscopic properties of
ferrous nitrosylated haem–human serum albumin (ferrous
HSA–haem-NO) is reported.
MATERIALS AND METHODS
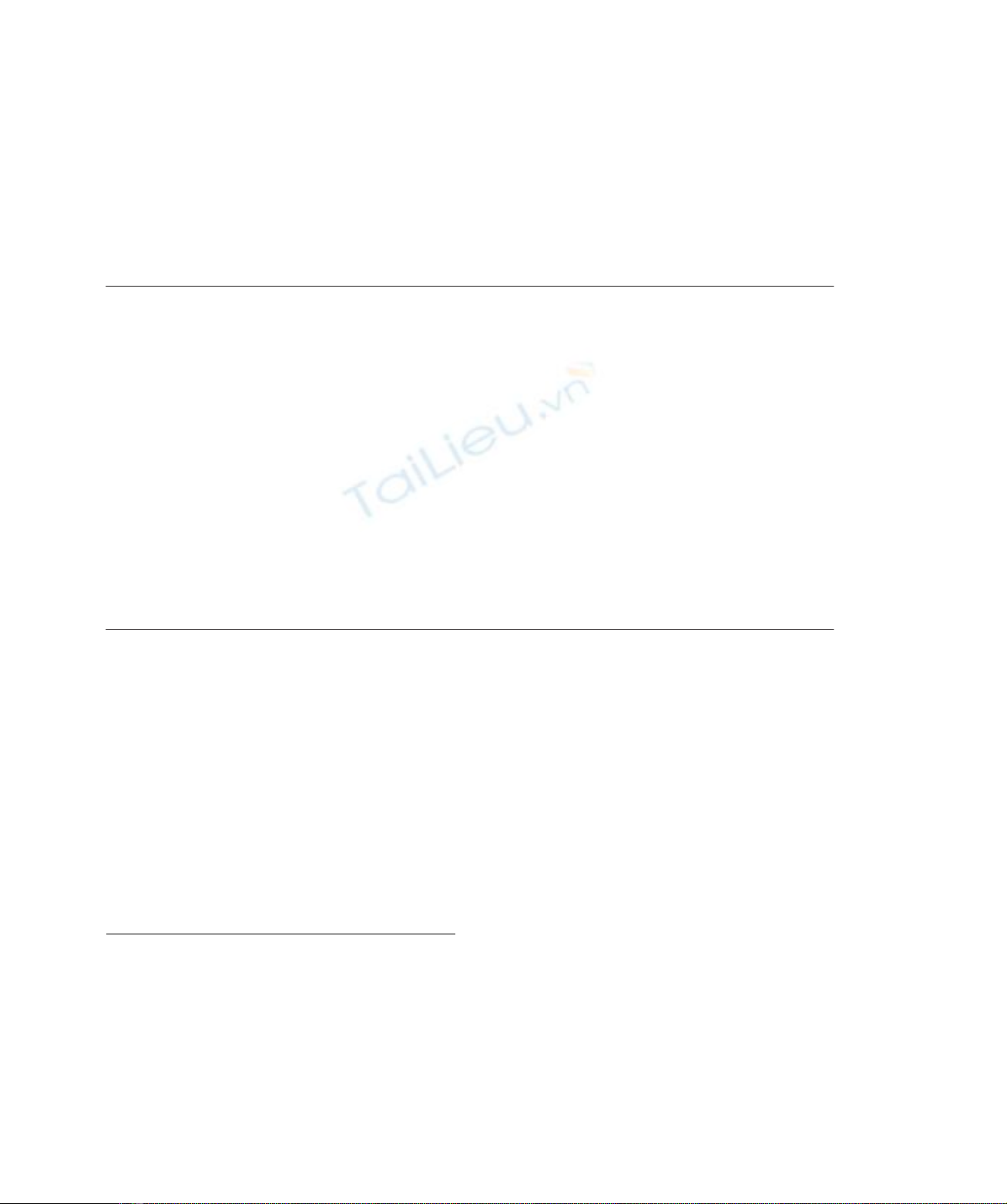
HSA, haem chloride, ibuprofen (Fig. 1), warfarin (see
Fig. 1), and the NO-donor S-nitroso-N-acetylpenicillamine
were from Sigma. Gaseous NO was purchased from Aldrich
Chemical Co. All the other products were from Merck AG.
All chemicals were of analytical or reagent grade and used
without further purification.
Ferric HSA–haem was prepared by adding 0.83-Mdefect
of ferric haem, dissolved in 1.0 10
21
MKOH, to an HSA
solution, in 1.0 10
21
Mphosphate buffer plus
1.0 10
21
MNaCl, pH 7.0 [8,21]. Both in the absence
and presence of ibuprofen and warfarin, haem binds to
HSA mostly at the high affinity site and virtually no free
haem is present in solution (see [1,8,21], and present study).
Values of the apparent dissociation equilibrium constant
(K
d
) for haem binding to the HSA primary site are
1.3 ^0.2 10
28
Min the absence of drugs, and
1.5 ^0.2 10
27
Min the presence of ibuprofen and
warfarin (5.0 10
22
M) (see [1,22,23] and the present
study).
Ferrous HSA–haem-NO was obtained under anaerobic
conditions: (a) by sequential addition of a 10-Mexcess
of sodium dithionite or sodium ascorbate and a 4-Mexcess of
KNO
2
to ferric HSA–haem, in 1.0 10
21
Mphosphate
buffer plus 1.0 10
21
MNaCl, pH 7.0; (b) by blowing
purified NO over the ferric HSA– haem solution
(1.0 10
21
Mphosphate buffer plus 1.0 10
21
MNaCl,
pH 7.0), in the absence and presence of a 5-Mexcess of
sodium dithionite or sodium ascorbate; and (c) by sequential
addition of a 10-Mexcess of dithiothreitol and a 4-Mexcess
of S-nitroso-N-acetylpenicillamine (which releases NO) to
ferric HSA – haem, in 1.0 10
21
Mphosphate buffer plus
1.0 10
21
MNaCl, pH 7.0 [8,21].
HSA solutions were prepared by dissolving the protein in
1.0 10
21
Mphosphate buffer plus 1.0 10
21
MNaCl, at
pH 7.0 and 25.0 8C. Haem solutions were prepared in
1.0 10
21
MKOH. Ibuprofen solutions were prepared by
dissolving the drug in 1.0 10
21
Mphosphate buffer plus
1.0 10
21
MNaCl, at pH 7.0 and 25.0 8C. Warfarin
solutions were prepared by stirring the drug in 1.0 10
21
M
phosphate buffer plus 1.0 10
21
MNaCl at pH 12.0
until it dissolved, then adjusting to pH 7.0 with HCl (at
25.0 8C).
Binding of ibuprofen and warfarin to ferric HSA – haem
was followed by electronic absorption spectroscopy at
pH 7.0, in 1.0 10
21
Mphosphate buffer plus
1.0 10
21
MNaCl, and 25.0 8C. Electronic absorption
spectra of ferric HSA–haem (5.0 10
26
Mto
2.0 10
24
M), in the absence and presence of ibuprofen
and warfarin (1.0 10
24
Mto 5.0 10
22
M), were
collected between 350 nm and 700 nm. The electronic
absorption spectra were recorded in 1-mm to 1-cm path
length cuvettes. The ibuprofen- and warfarin-induced
electronic absorption spectroscopic transition of ferric
HSA–haem was complete within the time to achieve
the sample preparation (,10 min). Test measurements
performed after 2 h excluded slow kinetic effects.
Fig. 1. Chemical structures of ibuprofen and warfarin.
qFEBS 2001 Drug binding to HSA–haem (Eur. J. Biochem. 268) 6215
Binding of ibuprofen and warfarin to ferric HSA – haem
was also followed by
1
H-NMR relaxometry at pH 7.0
(1.0 10
21
Mphosphate buffer plus 1.0 10
21
MNaCl)
and 25.0 8C.
1
H-NMR relaxometry of ferric HSA–haem
(1.0 10
23
M) in the absence and presence of ibuprofen
and warfarin (1.0 10
24
Mto 1.0 10
21
M) was investi-
gated on a Stelar SpinMaster Spectrometer (Stelar S.n.c.,
Mede, PV, Italy). Water proton relaxation rate (R
1
)
measurements were obtained at 0.47 T (i.e. at 20 MHz
proton Larmor frequency) by means of the Inversion-
Recovery technique (16 experiments, four scans). Magneti-
zation values were obtained by averaging the first 128 data
points of the Free Induction Decay. A phase cycle (1x, – x,
–x, 1x) was applied on the 908observation pulse to cut
off the y-scale receiver offset. A typical 908pulse width
was 3.5 ms. The
t
-values were increased linearly from a
starting value corresponding to one-seventh of the estimated
null-point (0.693/R
1
), so that the null-point occurs on
the middle of the inversion-recovery curve (seventh
experiment). In the 16th experiment the Free Induction
Decay is acquired after a single 908pulse, to obtain the M
1
value [24]. The reproducibility in R
1
measurements was
^0.5%. The temperature was controlled by a Stelar VTC-
91 airflow heater (Stelar S.n.c.), equipped with a copper-
constantan thermocouple; the actual temperature in the
probe head was measured with a Fluke 52k/j digital
thermometer (Fluke AG, Zu
¨rich, Switzerland), with an
uncertainty of ^0.3 8C. Values of the paramagnetic
contribution to the overall water solvent relaxation rate
(R
1p
) were determined by subtracting from the observed
relaxation rate (Robs
1) the blank relaxation rate value (Rdia
1)
measured for solutions containing HSA at the same
concentration without the paramagnetic prosthetic group.
Test measurements performed after 2 h excluded slow
kinetic effects [7].
Haem binding to HSA in the absence and presence of
ibuprofen and warfarin was followed by electronic
absorption spectroscopy (between 350 nm and 450 nm) at
pH 7.0 (1.0 10
21
Mphosphate buffer plus 1.0 10
21
M
NaCl) and 25.0 8C [22]. The HSA concentration ranged
between 3.0 10
28
Mand 2.0 10
26
M, the haem
concentration was 1.0 10
27
M, and the ibuprofen and
warfarin concentrations were 5.0 10
22
M. The electronic
absorption spectra were recorded in a 10-cm path length
cuvette. The haem-induced electronic absorption spectro-
scopic transition of HSA was complete within the time
to achieve the sample preparation (,10 min). Test
measurements performed after 2 h excluded slow kinetic
effects.
Binding of ibuprofen and warfarin to ferrous HSA–haem-
NO was followed by X-band EPR spectroscopy at pH 7.0 in
1.0 10
21
Mphosphate buffer plus 1.0 10
21
MNaCl,
and 2173 8C. X-band EPR spectra of ferrous HSA–haem-
NO (3.0 10
24
M) in the absence and presence of
ibuprofen and warfarin (5.0 10
22
M) were collected on
a Bruker ESP 300 spectrometer, operating at 9.42 GHz
microwave frequency, 100 kHz field modulation, 20 mW
microwave power, and 0.10 mT modulation amplitude. The
ibuprofen- and warfarin-induced EPR-spectroscopic tran-
sition of ferrous HSA – haem-NO was complete within the
time to achieve the sample preparation (,10 min). Test
measurements performed after 2 h excluded slow kinetic
effects [8].
RESULTS AND DISCUSSION
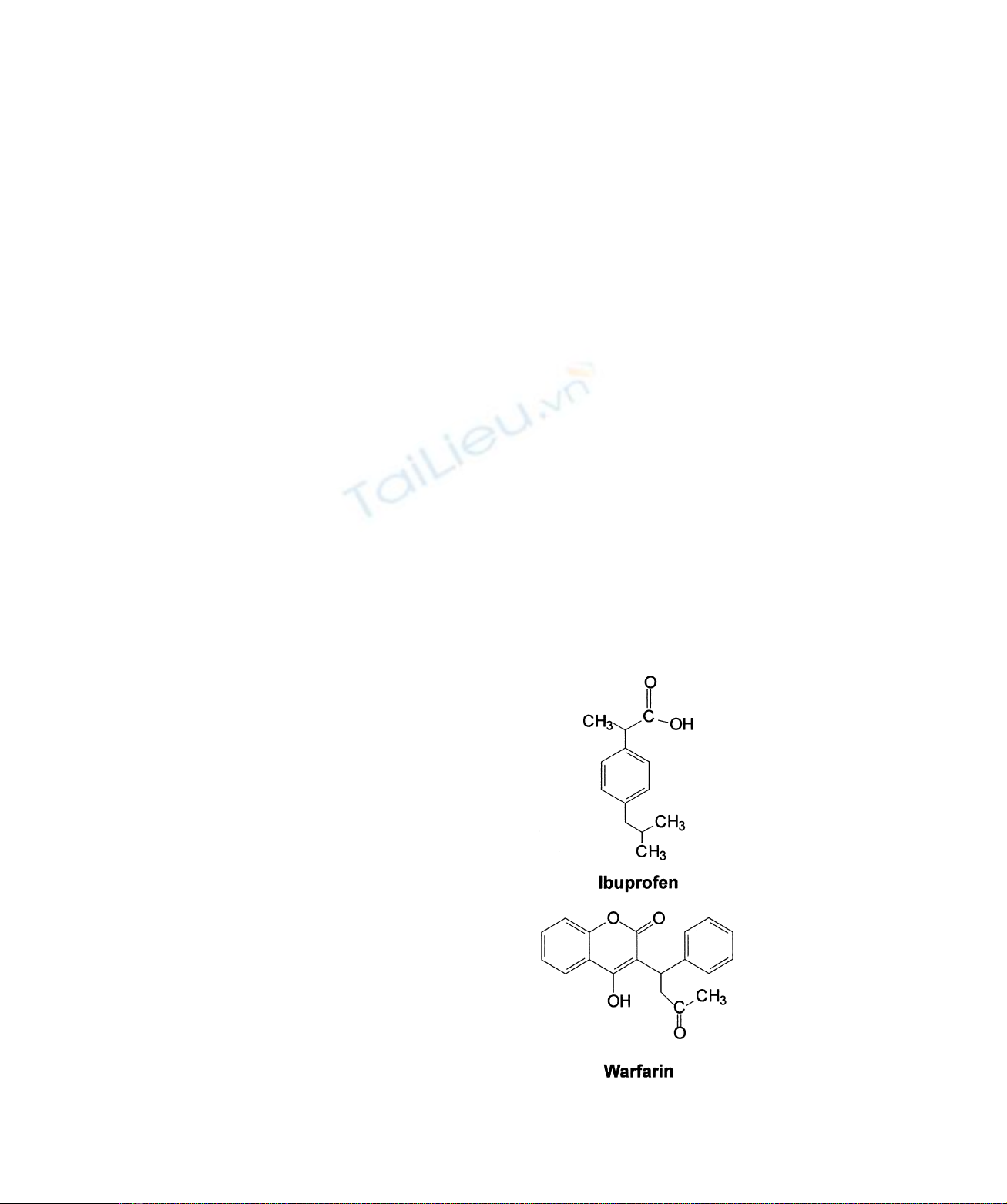
Fig. 2 shows the electronic absorption spectra of ferric
HSA–haem in the absence and presence of ibuprofen and
warfarin at pH 7.0 and 25.0 8C. Electronic absorbance
spectroscopy and
1
H-NMR relaxometry indicate that the
haem iron atom of ferric HSA –haem is hexa-coordinated,
possibly being bonded to His105 and Tyr148 as suggested
by docking simulations [7]. Upon drug binding, neither a
change in the haem-iron atom coordination number, nor in
the spin state of the metal centre, is observed. Spectra shown
in Fig. 2 are indicative of a high-spin state of the haem-iron.
Actually, even the minor low-spin component [22] seems to
diminish in the presence of either drug. In fact, the Soret
band is blue-shifted, the charge transfer band is red-shifted
and the aband is decreased in intensity with respect to the
bband. The spectral features shown in Fig. 2 are indicative
of a drug-dependent conformational transition(s) that does
not affect the inner coordination sphere of the haem iron
atom.
Fig. 2. Effect of ibuprofen and warfarin on the electronic
absorption spectroscopic properties of ferric HSA–haem. Elec-
tronic absorption spectra of ferric HSA–haem were obtained in the
absence (spectrum a) and in the presence of 5.0 10
22
Mibuprofen
(spectrum b, continuous line) and 5.0 10
22
Mwarfarin (spectrum b,
filled circles) at pH 7.0 and 25.0 8C. The electronic absorption spectra
of ferric HSA –haem in the presence of ibuprofen and warfarin are
superimposable. The ferric HSA–haem concentration was
8.4 10
26
M. The electronic absorption spectra were recorded in a
1-cm path length cuvette.
6216 S. Baroni et al. (Eur. J. Biochem. 268)qFEBS 2001
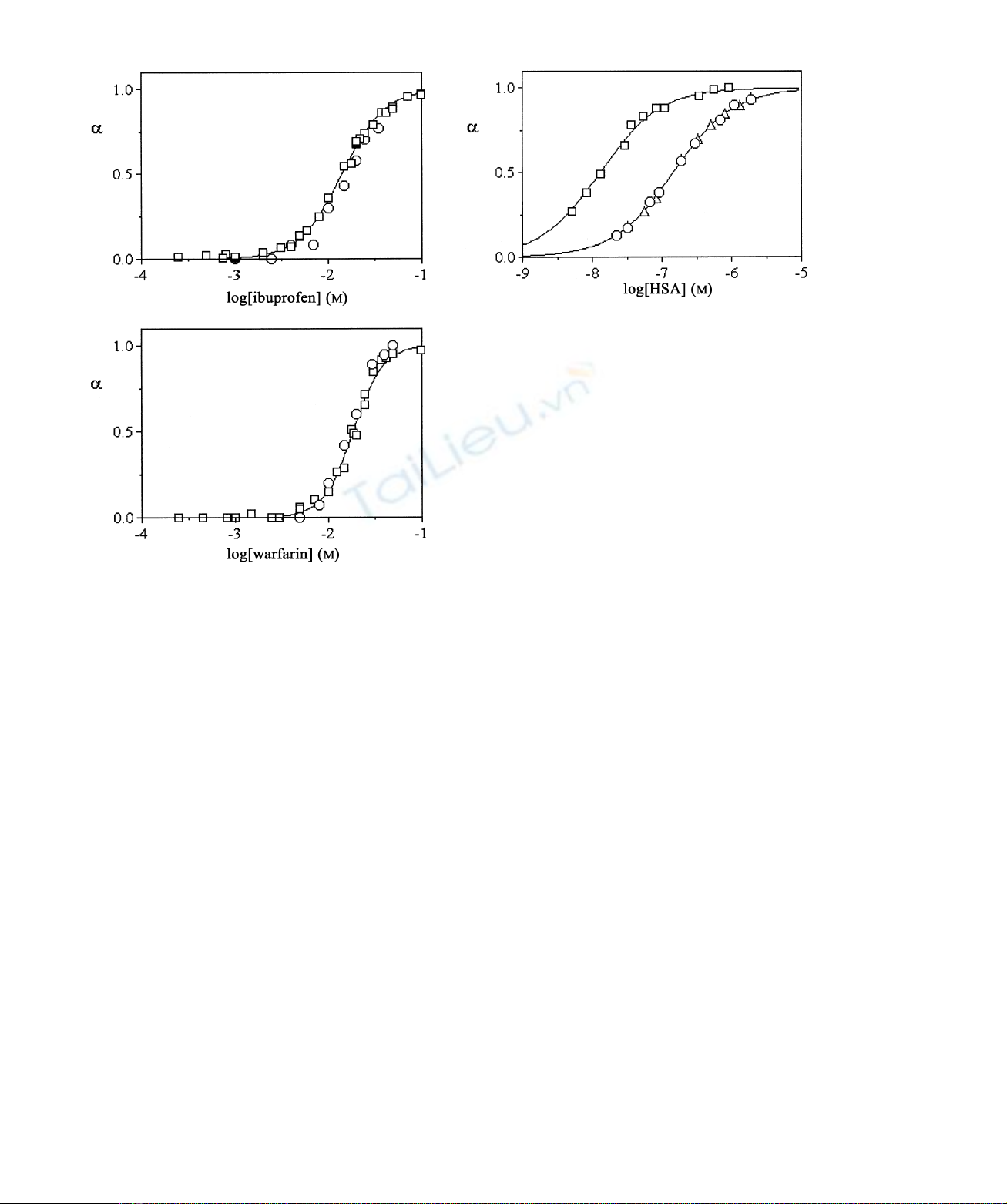
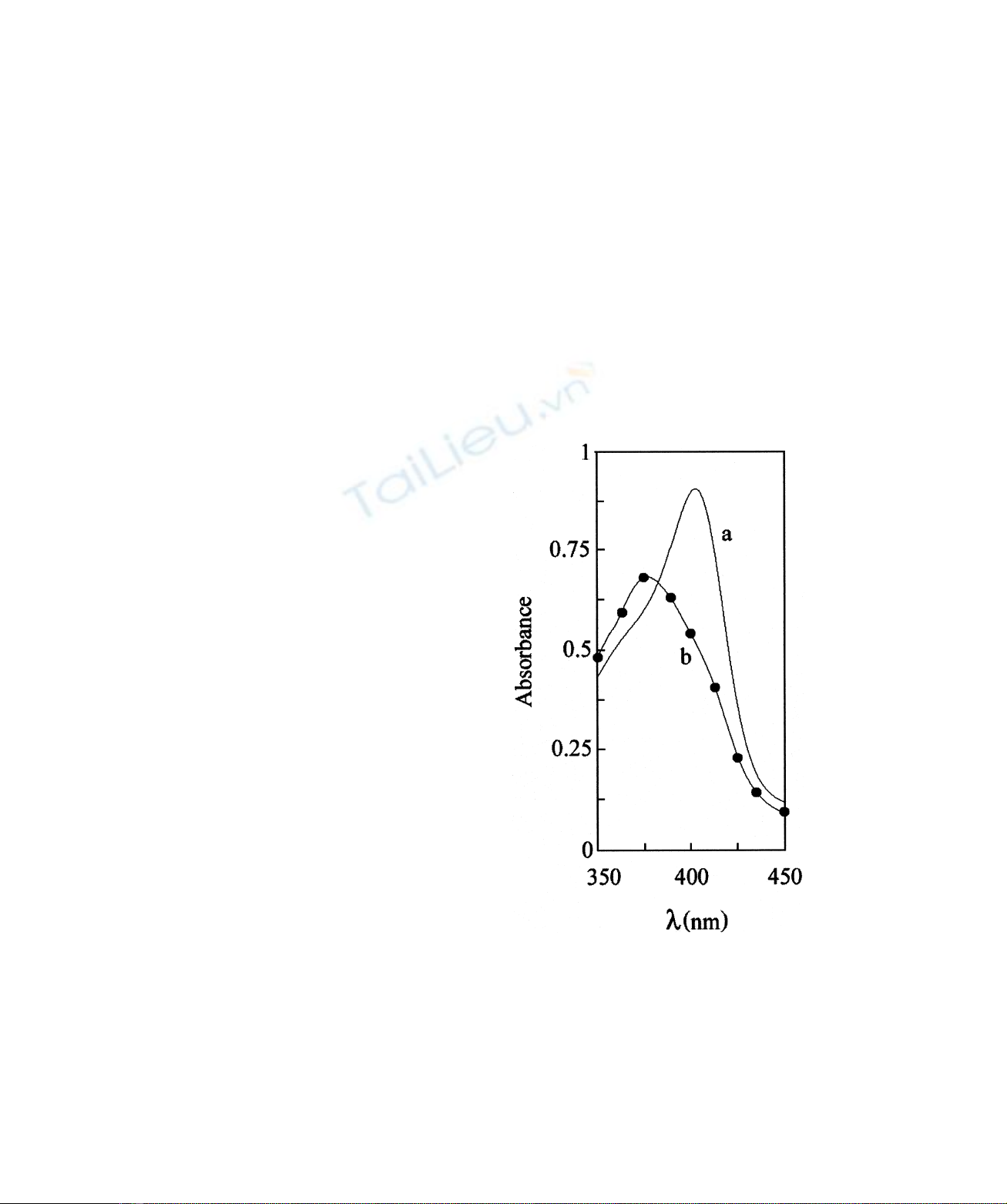
Fig. 3 shows the binding isotherms of ibuprofen and
warfarin to ferric HSA–haem, at pH 7.0 and 25.0 8C. Data
obtained by different techniques (i.e. electronic absorption
spectroscopy and
1
H-NMR relaxometry) are in good
agreement. By applying the minimum model accounting
for multiple binding sites per monomeric protein, a
relationship between the apparent equilibrium dissociation
constant (K
d
) for ibuprofen and warfarin binding to ferric
HSA–haem and the molar fraction of the ligand-bound
ferric HSA–haem (a) may be expressed according to
Eqn (1) [25]:
a¼ð½LT2½LbÞn/K
d1ð½LT2½LbÞn
fg
ð1Þ
where nis the Hill coefficient, and [L] is the ligand (i.e.
drug, HSA, HSA : ibuprofen, or HSA : warfarin) concen-
tration in the forms indicated by subscripts T (total) and b
(bound), respectively. [L]
b
was calculated according to
Eqn (2) [25]:
½Lb¼Kd1n:½QT1½LT2pðKd1n:½QT1½LTÞ2
24Kd:n:½QT:½LTgg/2ð2Þ
where [Q]
T
is the total ferric HSA–haem or haem
concentration.
The analysis of data given in Fig. 3 according to Eqn (1)
allowed the determination of values of K
d
(¼5.4 ^
1.1 10
24
M) and n(¼1.9 ^0.1) for ibuprofen binding
to ferric HSA–haem, and of K
d
(¼2.1 ^0.4 10
25
M)
and n(¼2.7 ^0.1) for ferric HSA – haem : warfarin
complex formation at pH 7.0 and 25.0 8C. The K
d
value
for ibuprofen binding to ferric HSA–haem is higher than
those reported for drug binding to the ibuprofen primary site
(K
d
¼3.7 10
27
M, at pH 7.4 and 37.0 8C) [1,15] and to
the ibuprofen secondary cleft (K
d
<410
25
Mat pH 7.4
and 37.0 8C) [1,15] of haem-free HSA. Also the K
d
value for
warfarin binding to ferric HSA–haem is higher than that
reported for drug binding to the warfarin primary site
(K
d
¼3.0 10
26
Mat pH 7.4 and 25.0 8C) [1,16 – 18] of
haem-free HSA.
Fig. 4 shows the binding isotherms of ferric haem to HSA
in the absence and presence of ibuprofen and warfarin, as
obtained by electronic absorption spectroscopy at pH 7.0
and 25.0 8C. Data analysis according to Eqn (1) allowed the
determination of values of the apparent dissociation
equilibrium constant (K
d
) and of the Hill coefficient (n)
for haem binding to HSA, in the absence and presence of
ibuprofen and warfarin. The K
d
value for ferric haem
binding to HSA, in the absence of drugs, is
1.3 ^0.2 10
28
Mat pH 7.0 and 25.0 8C. Remarkably,
the K
d
value for ferric haem binding to HSA determined
here (see Fig. 4) is in excellent agreement with that reported
in the literature (K
d
¼110
28
Mat pH 7.0 and 24.0 8C)
Fig. 3. Ibuprofen and warfarin binding to ferric HSA–haem.
Electronic absorption spectroscopic and
1
H-NMR relaxometric binding
isotherms of ibuprofen and warfarin to ferric HSA–haem were obtained
at pH 7.0 and 25.0 8C. Circles and squares indicate data obtained by
electronic absorption spectroscopy and
1
H-NMR relaxometry,
respectively. The continuous lines were obtained by using Eqn (1).
Best fitting parameters are K
d
¼5.4 ^1.1 10
24
Mand
n¼1.9 ^0.1 for ibuprofen binding, and K
d
¼2.1 ^0.4 10
25
M
and n¼2.7 ^0.1 for warfarin binding. The ferric HSA– haem
concentration was 1.5 10
24
Mand 1.0 10
23
Mfor electronic
absorption spectroscopic and
1
H-NMR relaxometric experiments,
respectively. The electronic absorption spectra were recorded in a
1-mm path length cuvette.
Fig. 4. Haem binding to HSA. Electronic absorption spectroscopic
binding isotherms of haem to HSAwere obtained in the absence (A) and
presence of 5.0 10
22
Mibuprofen (K) and 5.0 10
22
Mwarfarin
(W) at pH 7.0 and 25.0 8C. The continuous lines were obtained by using
Eqn (1). Best fitting parameters for ferric HSA– haem formation are
K
d
¼1.3 ^0.2 10
28
Mand n¼1.0 ^0.1, in the absence of drugs,
and K
d
¼1.5 ^0.2 10
27
Mand n¼1.0 ^0.1, in the presence of
ibuprofen and warfarin. The haem concentration was 1.0 10
27
M.
The electronic absorption spectra were recorded in a 10-cm path length
cuvette. For further details, see text.
qFEBS 2001 Drug binding to HSA–haem (Eur. J. Biochem. 268) 6217
[23]. In the presence of saturating amounts of ibuprofen and
warfarin (5.0 10
22
M) the affinity of haem for HSA
decreases by about one order of magnitude, the drug-
independent K
d
value being 1.5 ^0.2 10
27
Mat pH 7.0
and 25.0 8C. In the absence and presence of drugs, the value
of nfor haem binding to HSA is 1.0 ^0.1 at pH 7.0 and
25.0 8C (see Fig. 4).
Data reported in Figs 3 and 4 indicate that haem binding
to HSA inhibits ibuprofen and warfarin association to the
warfarin primary cleft (i.e. Sudlow’s site I), corresponding
to the ibuprofen secondary site [1,3,11]. Then, ibuprofen
and warfarin impair ferric HSA –haem formation. Remark-
ably, the ibuprofen primary cleft (i.e. Sudlow’s site II)
appears to be functionally and spectroscopically uncoupled
to the haem site of HSA.
Ferric HSA–haem has been widely investigated by
1
H-NMR relaxometry [7]. The high value of the paramagnetic
contribution to the water relaxation rate (R
1p
)of
hexacoordinated ferric HSA–haem (¼4.8 mM
21
:s
21
at
20 MHz, pH 7.2 and 25 8C) has been ascribed to the
occurrence of slowly exchanging water molecules in the
surroundings of the paramagnetic ferric haem center [7]. In
the presence of saturating amounts of ibuprofen and
warfarin, the R
1p
value of hexacoordinated ferric HSA–
haem decreases to 0.4 mM
21
:s
21
at 20 MHz, pH 7.0 and
25 8C (data not shown). The decrease of the R
1p
value upon
drug binding may reflect a conformational transition(s)
towards a ferric HSA–haem state where slowly exchanging
water molecules are far apart from the paramagnetic centre.
On the other hand, the lifetime of the ferric haem centre
hydration shell could be shortened to approach the diffusion
mean time [26–28].
Fig. 5 shows a ribbon diagram of HSA (PDB code 1E78)
[5]. Remarkably, a cavity hosting three water molecules can
be located at the interface between the haem cleft and the
warfarin primary site (i.e. Sudlow’s site I). These water
Fig. 5. Sudlow’s site I and haem cleft location
in HSA. Buried water molecules (blue spheres)
are located at the interface between the warfarin
primary site (i.e. Sudlow’s site I; highlighted
in green) and the haem cleft (traced in red).
The HSA backbone is rendered as a ribbon
model. HSA atomic coordinates were recovered
from the Protein Data Bank (PDB ID: 1E78) [19].
For details, see [5,7,10,14] and text.
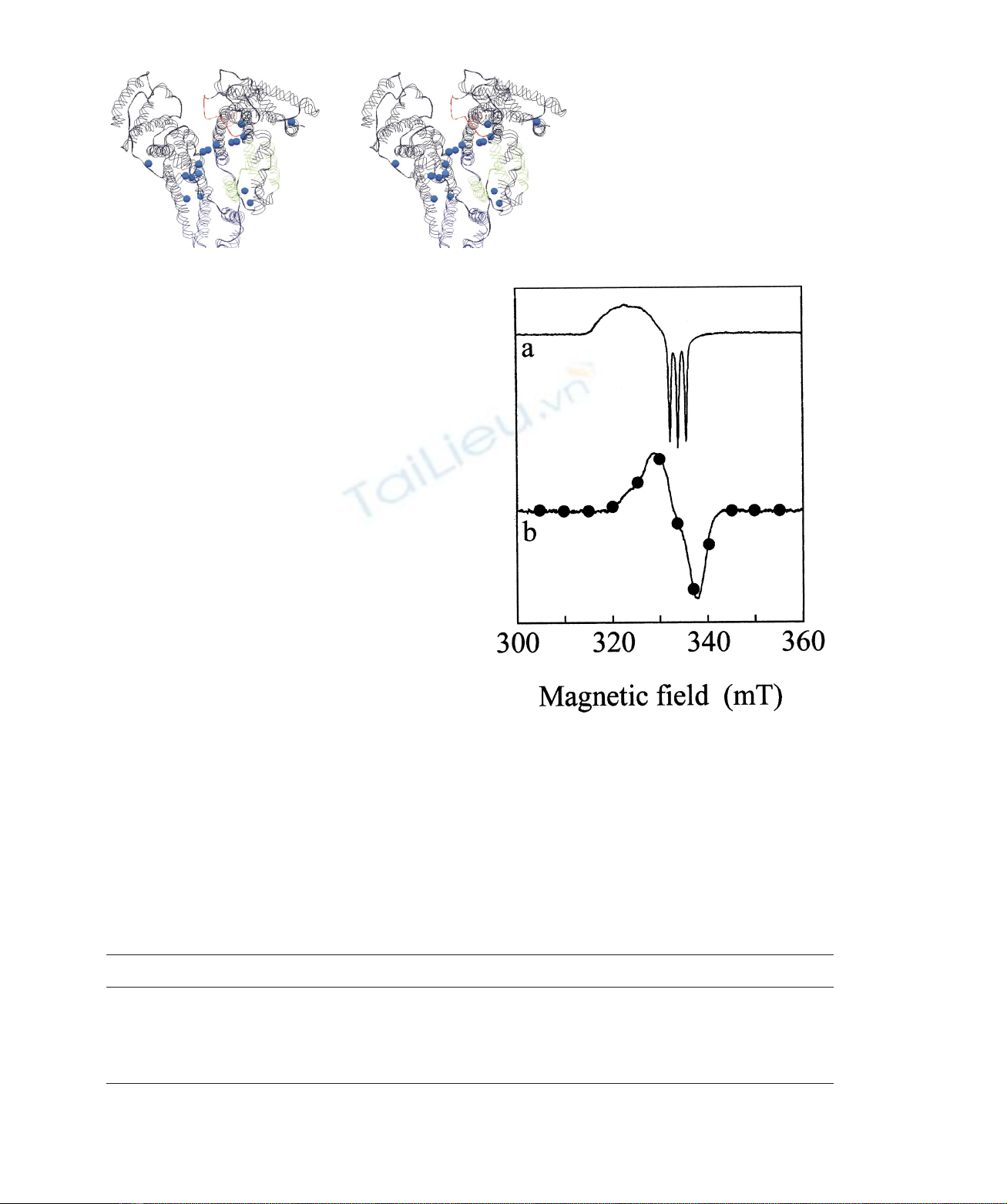
Fig. 6. Effect of ibuprofen and warfarin on the EPR spectroscopic
properties of ferrous HSA–haem-NO. X-band EPR spectra of ferrous
HSA– haem-NO were obtained in the absence (spectrum a) and in the
presence of 5.0 10
22
Mibuprofen (spectrum b, continuous line) and
5.0 10
22
Mwarfarin (spectrum b, filled circles) at pH 7.0 and
2173 8C. The X-band EPR spectra of ferrous HSA –haem-NO in the
presence of ibuprofen and warfarin are superimposable. The ferrous
HSA– haem-NO concentration was 3.0 10
24
M.
Table 1. X-band EPR parameters and the haem-iron coordination state of HSA –haem-NO. Values listed are for
14
N systems. Experimental
conditions were pH 7.0 and 2173 8C. US, unresolved signal.
Conditions A
3
(mT) g
1
g
2
g
3
Coordination state
Stripped
a
1.65 2.095 2.060 2.010 Five
Bezafibrate
b
US 2.064 1.983 2.005 Six
Clofibrate
b
US 2.064 1.983 2.005 Six
Ibuprofen
a
US 2.064 1.983 2.005 Six
Warfarin
a
US 2.064 1.983 2.005 Six
a
Present study.
b
From [8].
6218 S. Baroni et al. (Eur. J. Biochem. 268)qFEBS 2001


























