BchJ and BchM interact in a 1 : 1 ratio with the
magnesium chelatase BchH subunit of
Rhodobacter capsulatus
Artur Sawicki and Robert D. Willows
Department of Chemistry and Biomolecular Sciences, Macquarie University, Sydney, NSW, Australia
Keywords
bacteriochlorophyll biosynthesis; BchJ;
BchM; magnesium chelatase;
O-methyltransferase
Correspondence
R. D. Willows, Department of Chemistry
and Biomolecular Sciences Macquarie
University, NSW 2109, Australia
Fax: +6 298508245
Tel: +6 298508146
E-mail: robert.willows@mq.edu.au
Website: http://www.cbms.mq.edu.au
(Received 23 December 2009, revised 11
August 2010, accepted 9 September 2010)
doi:10.1111/j.1742-4658.2010.07877.x
Substrate channeling between the enzymatic steps in the (bacterio)chloro-
phyll biosynthetic pathway catalyzed by magnesium chelatase (BchI ⁄ChlI,
BchD ⁄ChlD and BchH ⁄ChlH subunits) and S-adenosyl-l-methionine:mag-
nesium-protoporphyrin IX O-methyltransferase (BchM ⁄ChlM) has been
suggested. This involves delivery of magnesium-protoporphyrin IX from
the BchH ⁄ChlH subunit of magnesium chelatase to BchM ⁄ChlM. Stimula-
tion of BchM ⁄ChlM activity by BchH ⁄ChlH has previously been shown,
and physical interaction of the two proteins has been demonstrated. In
plants and cyanobacteria, there is an added layer of complexity, as Gun4
serves as a porphyrin (protoporphyrin IX and magnesium-protoporphy-
rin IX) carrier, but this protein does not exist in anoxygenic photosynthetic
bacteria. BchJ may play a similar role to Gun4 in Rhodobacter, as it has
no currently assigned function in the established pathway. Purified recom-
binant Rhodobacter capsulatus BchJ and BchM were found to cause a shift
in the equilibrium amount of Mg-protoporphyrin IX formed in a magne-
sium chelatase assay. Analysis of this shift revealed that it was always in a
1 : 1 ratio with either of these proteins and the BchH subunit of the mag-
nesium chelatase. The establishment of the new equilibrium was faster with
BchM than with BchJ in a coupled magnesium chelatase assay. BchJ
bound magnesium-protoporphyrin IX or formed a ternary complex with
BchH and magnesium-protoporphyrin IX. These results suggest that BchJ
may play a role as a general magnesium porphyrin carrier, similar to one
of the roles of GUN4 in oxygenic organisms.
Structured digital abstract
lMINT-7994803:bchI (uniprotkb:P26239), bchD (uniprotkb:P26175)andbchH (uniprotkb: P26162)
physically interact (MI:0915)bycosedimentation in solution (MI:0028)
lMINT-7994743:bchJ (uniprotkb:P26169), bchI (uniprotkb:P26239), bchD (uniprotkb:P26175)
and bchH (uniprotkb:P26162)physically interact (MI:0915)by cosedimentation in solution (MI:0028)
lMINT-7994762:bchM (uniprotkb:P26236), bchH (uniprotkb:P26162), bchD (uniprotkb: P26175)
and bchI (uniprotkb:P26239)physically interact (MI:0915)bycosedimentation in solution
(MI:0028)
Abbreviations
BchM, S-adenosyl-L-methionine:magnesium-protoporphyrin IX O-methyltransferase; Mg-proto, magnesium-protoporphyrin IX;
proto, protoporphyrin IX; SAM, S-adenosyl-L-methionine.
FEBS Journal 277 (2010) 4709–4721 ª2010 The Authors Journal compilation ª2010 FEBS 4709
Introduction
Magnesium chelatase (EC 6.6.1.1) and S-adenosyl-l-
methionine:magnesium-protoporphyrin IX O-methyl-
transferase (BchM) (EC 2.1.1.11) catalyze sequential
steps of the (bacterio)chlorophyll biosynthetic pathway
[1,2]. Magnesium chelatase requires free magnesium
and ATP hydrolysis to convert protoporphyrin IX
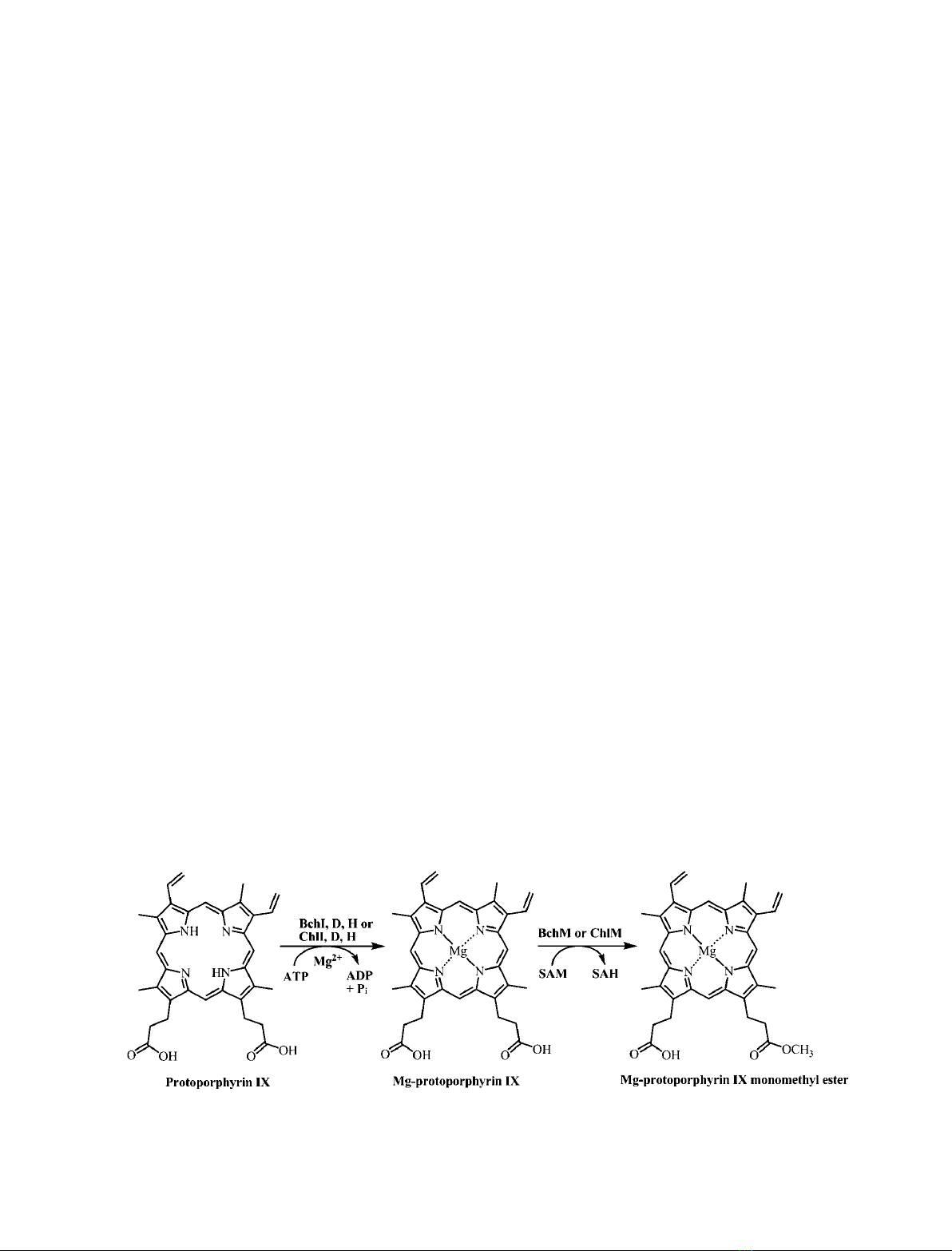
(proto) to magnesium-protoporphyrin IX (Mg-proto)
[3–5]. This is followed by O-methyltransferase using
the ubiquitous methylating molecule S-adenosyl-l-
methionine (SAM) to convert Mg-proto to Mg-proto
monomethyl ester [6] (Fig. 1). Magnesium chelatase is
the more complex of the two enzymes, being composed
of at least three subunits. It consists of BchI, BchD
and BchH subunits in bacteriochlorophyll biosynthetic
bacteria, or ChlI, ChlD and ChlH subunits in chloro-
phyll biosynthetic organisms, including bacteria, algae
and plants. There are differences in the complexity of
magnesium chelatase, depending on the organism. For
example, Arabidopsis thaliana has two ChlI isoforms
[7], with each protein contributing to magnesium
chelatase activity and complex formation [8–10]. The
green sulfur bacterium Chlorobaculum tepidum has the
unique property of having three isoforms of BchH,
with suggestions of regulatory properties of at least
one subunit [11]. BchI and BchD hexamers [12,13]
form a symmetrically stacked BchID complex, with
each subunit composed of a trimer of dimers [12], and
BchD providing a stable platform for BchI [14]. The
largest subunit of magnesium chelatase, BchH, con-
tains bound proto [15–17] and transiently interacts
with the BchID complex [18]. This interplay initiates a
burst of ATPase activity supplied by BchI, which
drives magnesium chelation to form Mg-proto [18,19].
It has been shown in plants (A. thaliana) and cyano-
bacteria (Synechocystis) that a subsidiary porphyrin-
binding protein, Gun4, associates with ChlH and
increases magnesium chelatase activity [20–22]. This
interaction was recently localized at the chloroplast
membranes [23], which is consistent with the findings
of single-protein studies showing both Gun4 and ChlH
existing in the stroma and membrane [20,24,25].
Currently, there is no identified homolog of Gun4 in
anoxygenic photosynthetic bacteria, and it was sug-
gested that BchJ, a protein that is absent in plants,
algae and most bacteria, could play a similar role to
Gun4 in purple (sulfur and nonsulfur) bacteria and
green sulfur bacteria [1]. This was postulated because
BchJ lacks 8-vinyl reductase activity [1], contradicting
the conclusions drawn from previous mutational
studies of bchJ [26]. However, the role of BchJ
has not been studied, so it has no classified role in
bacteriochlorophyll biosynthesis.
O-methyltransferase was thoroughly characterized
biochemically in the 1970s to 1980s [27–29], with stud-
ies also being conducted on highly purified His-tagged
enzymes from Synechocystis [30,31], Rhodobacter
capsulatus [32] and C. tepidum [11]. The enzyme is
membrane-associated [33] and is a high molecular mass
polymer in anaerobic bacteria [11,32], whereas the
cyanobacterial form is soluble as a monomer [30]. The
recent report showing the potential of a continuous
coupled colorimetric assay for SAM-dependent methyl-
transferase enzymes [34] was validated with ChlM of
Synechocystis, yielding kinetic constants comparable to
those found in previous uncoupled HPLC-based
stopped assays for O-methyltransferase [35]. Other
work has shown an inextricable link between folate
biosynthesis and O-methyltransferase activity, which is
attributable to the C1 pathway and SAM ⁄S-adenosyl-
l-homocysteine (SAH) metabolic levels [36].
The interaction between magnesium chelatase and
O-methyltransferase has been known for over 35 years,
through initial experiments using whole cells of Rho-
dobacter sphaeroides [37]. However, the precise nature
Fig. 1. Magnesium chelatase and O-methyl transferase reactions.
Interaction of BchM ⁄BchJ with magnesium chelatase A. Sawicki and R. D. Willows
4710 FEBS Journal 277 (2010) 4709–4721 ª2010 The Authors Journal compilation ª2010 FEBS

of the interaction was not apparent until much later,
particularly because of the complexity of magnesium
chelatase. A breakthrough occurred with the discovery
that BchH is the porphyrin-binding subunit, with a
1 : 1 molar ratio of protein ⁄porphyrin [17]. As this
subunit shares the ability to bind Mg-proto with
O-methyltransferase, interactions between BchH ⁄ChlH
of magnesium chelatase and O-methyltransferase may
be possible. Studies with Synechocystis showed sub-
stantial stimulation of O-methyltransferase activity
with ChlH, up to 10-fold on a millisecond timescale
[38]. A 1.3–1.6-fold increase in O-methyltransferase
activity was also found with BchS and BchT isoforms
of BchH from C.tepidum [11]. BchH from this species
actually reduced O-methyltransferase activity, prompt-
ing the idea that it could be involved in regulation
[11]. Some evidence also exists for BchH–BchM inter-
actions in R. capsulatus [39]; however, crude protein
preparations were used in this study, and the precise
molecular interaction could not be deduced. Our
previous findings showed no distinct impact of
O-methyltransferase activity upon addition of various
magnesium chelatase subunits, including the fully func-
tional BchIDH complex [32]. In tobacco plants that
were transgenic for sense or antisense ChlM, magne-
sium chelatase and O-methyltransferase activities and
protein expression were found to be in a constant
ratio, and an interaction was demonstrated between
ChlM and ChlH [40,41].
In this article, we present further evidence highlight-
ing the interaction between magnesium chelatase,
BchM and BchJ from the purple nonsulfur bacterium
R. capsulatus. Our results predominantly focus on the
shift in magnesium chelatase reaction equilibria of
proto ⁄Mg-proto upon addition of BchM or BchJ. We
provide kinetic evidence of a direct interaction between
BchM or BchJ and the BchH Mg-proto subunit of
magnesium chelatase, and show a concentration-depen-
dent effect. Our data also suggest that BchJ may serve
as an Mg-proto carrier between the BchH Mg-proto
subunit and BchM.
Results and Discussion
Physical and structural properties of BchJ
His-tagged BchJ was readily purified to homogeneity
in a single step by metal affinity chromatography
(Fig. S1). BchJ (22 kDa) behaved as a high molecular
mass complex (> 200 kDa) in size-exclusion chroma-
tography, even in the presence of the detergent Tri-
ton X-100 and dithiothreitol (Fig. S2). It has already
been shown, by gel filtration, that BchM exists as a
high molecular mass multimer in both purple nonsul-
fur and green sulfur bacteria [11,32], and this is differ-
ent to the situation with ChlM from Synechocystis,
which exists as a monomer [30].
Despite the large aggregates, BchM is enzymatically
active (Fig. S3), and CD spectroscopy of BchM and
BchJ indicates that they have similar and well-defined
secondary structure compositions, with the majority of
the structure being a-helical (52–53%). There is a rela-
tively large proportion of unordered structure
(19% ⁄25%) for BchJ ⁄BchM, and BchJ has a higher
proportion of b-strands than BchM (20.8% ⁄9.1%)
(Fig. 2 and Table S1).
Effect of magnesium on BchM and BchJ, and
their interactions with magnesium chelatase
As coupled magnesium chelatase assays with BchM or
BchJ require free magnesium, we first determined
A
B
Fig. 2. CD spectra of BchM or BchJ with Mg-proto. Experiments
were performed in 10 mMsodium phosphate (pH 7.6), 40 lMTri-
cine ⁄NaOH (pH 8.0) and 8.6 mMglycerol. The concentrations
of BchM, BchJ and Mg-proto (where used) were all 400 nM. The
x-axis is divided into two sections for clarity, with the left-hand side
providing secondary structural information on the protein, and the
right-hand side depicting any interaction of protein with Mg-proto in
the Soret region. (A) BchM alone (open circles) or BchM together
with Mg-proto (closed circles). (B) BchJ alone (open circles) or BchJ
together with Mg-proto (closed circles).
A. Sawicki and R. D. Willows Interaction of BchM ⁄BchJ with magnesium chelatase
FEBS Journal 277 (2010) 4709–4721 ª2010 The Authors Journal compilation ª2010 FEBS 4711

whether magnesium would be detrimental to BchM or
BchJ individually in terms of solubility. Free magne-
sium above 2mmcaused substantial precipitation of
BchM, whereas BchJ was mildly affected at 8mm
(Fig. 3A). In terms of their effect on magnesium
chelatase, BchM, BchJ and the detergent Tween-80 all
increased the product formation of magnesium
chelatase in a similar manner at concentrations of free
magnesium above 2 mm. BchM also showed this effect
at the lower free magnesium concentrations tested
(0.78 and 1.2 mm) (Fig. 3B). The aggregation of BchM
in this study could explain the previously observed
adverse effect of magnesium on O-methyltransferase
activity (65% reduction at 10 mm) [32]. The apparent
stimulatory effect of BchM, BchJ or Tween-80 on
magnesium chelation (Fig. 3B) in a 90 min stopped
assay may be attributable to stabilization of the
Mg-proto product and ⁄or interaction with one or
more of the magnesium chelatase subunits.
Using the aggregation of BchM or BchJ to
coprecipitate magnesium chelatase subunits
We further analyzed interactions of magnesium chela-
tase with BchM or BchJ by looking at the distribution
of proteins into soluble and insoluble fractions. It
should be noted that the supernatant fractions
(Fig. 4A) had one-sixth of the relative loading of the
pellet fractions (Fig. 4B). At the magnesium concentra-
tion used, BchH was soluble, BchM was largely insolu-
ble, and BchJ existed in both the soluble and insoluble
fractions (Fig. 4A,B). Addition of BchM or BchJ to
magnesium chelatase significantly increased the pro-
portion of BchH in the insoluble fraction. There was a
less distinct increase in BchI and BchD in the insoluble
fraction, suggesting that there is a physical interaction
between BchH and BchM or BchH and BchJ.
Spectroscopic analysis of BchM or BchJ with
Mg-proto and proto; Soret and far-UV changes
BchH can bind proto and Mg-proto [15–17], so we
tested the ability of BchM or BchJ to bind proto and
Mg-proto, because this may provide more information
on BchM ⁄BchJ–BchH interactions. Both BchM and
BchJ could bind proto and Mg-proto in vitro,as
determined with absorbance spectroscopy (Fig. 5). The
porphyrin Soret peaks of Mg-proto and proto were
red-shifted, by 10 and 25 nm, respectively, when
they were added to BchM or BchJ. A similar shift was
observed with Tween-80 but not with an alterative
protein such as aldolase. The similar spectral changes
induced by Tween-80, BchM and BchJ indicate a
change in the environment of proto ⁄Mg-proto similar
to micellar Tween-80, implying that the porphyrins
bind in a hydrophobic environment on these proteins.
The shift in the Soret peak is often characteristic of a
nonplanar distortion of the porphyrins [42]. Distortion
A
B
Fig. 3. Precipitation of BchM or BchJ with magnesium, and the
effect of these conditions on magnesium chelatase equilibria. All
experiments contained final concentrations as described for magne-
sium chelatase assays, except for 3.2 mMdithiothreitol, 0.5 mM
ATP, and variable free magnesium: 0.38, 0.78, 1.2, 2.0, 2.9, 3.7,
7.9 and 12 mM. Where stated, 87 nMBchH–proto, 850 nM
BchM ⁄BchJ, and 64 lMTween-80 were also included. (A) Bradford
assays of BchM or BchJ with increasing magnesium concentra-
tions. Assays were performed by adding the following components:
3.6 lL of BchID buffer (assay buffer with 4.4 mMdithiothreitol,
10 mMurea, 3.5 mMglycerol), 23.8 lL of MgATP (assay buffer
with 4.4 mMdithiothreitol, and variable MgCl
2
concentrations: 0,
0.96, 1.93, 3.8, 5.8, 7.7, 17 and 27 mM) and 27.5 lL of either
BchM (open circles) or BchJ (closed circles). Samples were centri-
fuged (18 000 gfor 7 min at room temperature), and the superna-
tant was added to Bradford reagent (Bio-Rad) and compared with a
standard curve (BSA) to generate the final concentrations on the
y-axis. (B) Magnesium chelatase assays, with the y-axis represent-
ing the total Mg-proto produced at equilibrium. BchID (13.3 lL)
was refolded in assay buffer as stated above, and diluted to 100 lL
with variable concentrations of MgATP; this was followed by addi-
tion of 100 lL of BchH–proto together with either buffer alone
(50 mMTricine ⁄NaOH, pH 8.0, 19 mMglycerol, 2 mMdithiothreitol)
(open squares), BchM in buffer (open circles), BchJ in buffer
(closed circles) or Tween-80 in buffer (closed diamonds).
Interaction of BchM ⁄BchJ with magnesium chelatase A. Sawicki and R. D. Willows
4712 FEBS Journal 277 (2010) 4709–4721 ª2010 The Authors Journal compilation ª2010 FEBS

of the tetrapyrrole nucleus is common in nature
[42], being most notably seen with many heme (iron
tetrapyrrole)-binding proteins, but also with nickel
porphyrin-binding protein (cofactor F
430
) and bacterio-
chlorophyll-binding photosynthetic reaction centers.
We further tested the visible and far-UV properties
of BchM and BchJ, and with Mg-proto bound, using
CD (Fig. 2). BchM showed changes in the Soret region
of Mg-proto when bound to BchM, but there was no
obvious change in the far-UV region (Fig. 1 and
Table S1). The differences in the Soret region upon
Mg-proto binding to BchM signifies disymmetric
changes in porphyrin planarity. Therefore, it is sug-
gested that binding of Mg-proto to BchM distorts the
planarity of Mg-proto, causing the prominent signal in
the Soret CD region, similar to what is observed when
apomyoglobin is reconstituted with hemin [43]. In con-
trast, BchJ showed no structural changes upon binding
Mg-proto in the Soret region; however, significant
differences were observed in the CD spectra of
the far-UV region. The changes in the far-UV region
were seemingly inversely related, with a decrease in
b-strands (20.8% to 6.2%) being balanced by an
increase in regular a-helices (34% to 47%) (Table S1).
Thus, in comparison with BchM, BchJ showed a com-
paratively malleable secondary structure, with the
changes presumably being needed to accommodate
Mg-proto, as the porphyrin did have an induced chirality
detectable by CD when bound to the BchJ. This suggests
that the binding is specific and strains the porphyrin
in a similar manner as observed for myoglobin [43].
Interaction between magnesium chelatase and
BchM, BchJ or Tween-80
Previous findings have consistently shown that the
BchH subunit of magnesium chelatase enhances O-
methyltransferase activity in C. tepidum,Synechocystis,
R. capsulatus and tobacco [11,38–40,44], implying that
O-methyltransferase accepts the Mg-proto substrate
when bound to BchH. We found that BchM could uti-
lize Mg-proto produced in situ from BchH, with Mg-
proto monomethyl ester being made in the complete
coupled assay (BchIDHM SAM) (Fig. S3). Mg-proto
remains tightly bound to R. capsulatus BchH in the
magnesium chelatase reaction [15], and exchanges
slowly with exogenous proto [18]. BchM converts all of
the Mg-proto produced in a magnesium chelatase reac-
tion into the methyl ester (Fig. S3), and also shifts the
magnesium chelatase equilibrium, whether or not
methylation occurs (Figs 6, 7 and 8). To do both,
BchM must either release the Mg-proto from BchH or
stabilize the Mg-proto while it is bound to BchH. Dis-
tinguishing between these two possibilities with, for
example gel, filtration was not possible, owing to the
aggregation of BchM in magnesium chelatase assay
buffer. To more accurately quantify the respective
interactions of BchM, BchJ or Tween-80 with BchH,
we focused on their kinetic effects in a magnesium
chelatase assay, because our previous findings could
not establish a unique stimulatory effect of magnesium
chelatase subunits on O-methyltransferase activity [32].
It was initially found that BchM, BchJ or Tween-80
generated an increased amount of Mg-proto produced
by magnesium chelatase over time, and the time taken
to reach equilibrium was significantly faster for BchM
than for BchJ and Tween-80 (Fig. 6). Our subsequent
A
B
Fig. 4. Coprecipitation of magnesium chelatase subunits with
BchM or BchJ; distribution of protein into soluble and insoluble
fractions. M refers to the Bio-Rad broad-range ladder, with molecu-
lar masses in kilodaltons listed on the left-hand side. 1, BchIDH;
2, BchIDHM; 3, BchIDHJ; 4, BchIDH and Tween-80. Assays were
performed as described for magnesium chelatase, with final con-
centrations of 86 mMglycerol, 18 mMurea, 4 mMdithiothreitol,
0.33 lMBchD, 0.66 lMBchI, 1.385 lMBchH–proto and, if present,
2.7 lMBchM ⁄BchJ or 117 lMTween-80 in a total volume of
160 lL. The total assay time was 90 min; the assay was followed
by centrifugation at 18 000 gfor 7 min at room temperature to sep-
arate supernatant and pellet fractions. A total volume of 25 lLof
supernatant was loaded onto a 4–15% polyacrylamide gel (Bio-Rad)
(A). The pellet fraction was further washed twice 100 lL of assay
buffer without protein or detergent, vortexed for 10 s, and centri-
fuged after each wash step with the supernatant of each wash dis-
carded. The final pellet was resuspended in 25 lL of SDS ⁄PAGE
loading buffer (Nu-Sep), and the total volume was loaded onto the
gel (B). The approximate position of each protein is listed on the
right-hand side.
A. Sawicki and R. D. Willows Interaction of BchM ⁄BchJ with magnesium chelatase
FEBS Journal 277 (2010) 4709–4721 ª2010 The Authors Journal compilation ª2010 FEBS 4713


























