
Hyperthermal stability of neuroglobin and cytoglobin
Djemel Hamdane
1
, Laurent Kiger
1
, Sylvia Dewilde
2
, Julien Uzan
1
, Thorsten Burmester
3
,
Thomas Hankeln
4
, Luc Moens
2
and Michael C Marden
1
1 Inserm U473, Le Kremlin-Bice
ˆtre, France
2 Department of Biomedical Sciences, University of Antwerp, Belgium
3 Institute of Zoology, Johannes Gutenberg University of Mainz, Germany
4 Institute of Molecular Genetics, Johannes Gutenberg University of Mainz, Germany
Neuroglobin (Ngb) and cytoglobin (Cygb) have been
identified recently [1–3] as new globins in vertebrates.
Ngb is predominately expressed in certain regions of
the brain as well as in some endocrine tissues [1,4] and
at a higher level in the retina [5], whereas Cygb is
expressed in all tissues. Although sequence analysis
reveals little similarity with hemoglobin (Hb) and myo-
globin (Mb), Ngb and Cygb share many of the charac-
teristics of the globins such as reversible oxygen
binding and the overall three-dimensional globin fold
[6–8]. In the absence of external ligands such as oxy-
gen, Ngb and Cygb are hexa-coordinated with a bis-
histidyl heme [9].
The primary functions of Ngb and Cygb remain
unknown, but some hypotheses have been suggested
[9–11]. Expression of Ngb increases in cultures of neu-
rons under hypoxic conditions, and it appears that Ngb
may protect cells against hypoxia [12,13]. Thus Ngb
may have a Mb-like function, supplying the respiratory
chain of neuronal mitochondria with O
2
[1,5]. Cygb
may be a signaling or sensor protein [14], and may be
involved in collagen synthesis or in the protection
against reactive oxygen species [15]. Other physiological
roles, however, such as electron transfer, peroxidase
activity, NO binding or NO detoxification, as observed
for other hemoproteins, are still conceivable.
Unique in the globin family, Ngb and Cygb possess
cysteine residues capable of forming a disulfide bond
[16]. Reduction of the disulfide bond in Ngb increases
the affinity for the distal histidine by a factor of nearly
Keywords
cytoglobin; disulfide bond; ligand kinetics;
neuroglobin; protein melting temperature
Correspondence
M. C. Marden, Inserm U473, 78 rue du
General Leclerc, 94275 Le Kremlin-Bice
ˆtre,
France
E-mail: marden@kb.inserm.fr
(Received 6 December 2004, revised 14
February 2005, accepted 1 March 2005)
doi:10.1111/j.1742-4658.2005.04635.x
Neuroglobin (Ngb) and cytoglobin (Cygb), recent additions to the globin
family, display a hexa-coordinated (bis-histidyl) heme in the absence of
external ligands. Although these proteins have the classical globin fold they
reveal a very high thermal stability with a melting temperature (T
m
)of
100 C for Ngb and 95 C for Cygb. Moreover, flash photolysis experi-
ments at high temperatures reveal that Ngb remains functional at 90 C.
Human Ngb may have a disulfide bond in the CD loop region; reduction
of the disulfide bond increases the affinity of the iron atom for the distal
(E7) histidine, and leads to a 3 C increase in the T
m
for ferrous Ngb. A
similar T
m
is found for a mutant of human Ngb without cysteines. Appar-
ently, the disulfide bond is not involved directly in protein stability, but
may influence the stability indirectly because it modifies the affinity of the
distal histidine. Mutation of the distal histidine leads to lower thermal sta-
bility, similar to that for other globins. Only globins with a high affinity of
the distal histidine show the very high thermal stability, indicating that sta-
ble hexa-coordination is necessary for the enhanced thermal stability; the
CD loop which contains the cysteines appears as a critical region in
the neuroglobin thermal stability, because it may influence the affinity of
the distal histidine.
Abbreviations
Cygb, cytoglobin; GdmCl, guanidinium chloride; Hb, hemoglobin; Mb, myoglobin; Ngb, neuroglobin; T
m
, melting temperature.
2076 FEBS Journal 272 (2005) 2076–2084 ª2005 FEBS
10; it has therefore been hypothesized that reduction
of the SS bridge may promote oxygen release [16].
Proteins, and particularly enzymes, are generally
quite sensitive to environmental changes, e.g. elevated
temperatures, due to highly cooperative unfolding [17].
However, there are some exceptions such as those
found in extreme thermophilic microorganisms. Com-
parison of the protein structure from mesophiles and
thermophiles has allowed some explanation of thermo-
stability based on small solvent-exposed surface area
[18], increased packing density [19–21], core hydro-
phobicity [22], decreased surface loop length [21], and
the generation of salt bridges or hydrogen bonds
betweens polar residues [23–25]. The affinity of apo-
globin for heme or the orientation of the heme in the
pocket cavity may play a major role in the stability of
the holoprotein [26]. In general, few proteins are stable
above 80 C; examples are calcium-binding proteins
such as calmodulin or troponin C with T
m
>90C
for the Ca-bound form [27].
Results
Spectroscopy
The visible spectrum of dithionite-reduced Ngb, Cygb,
and Drosophila Hb showed characteristic absorption
maxima of hexa-coordinated (bis-histidyl) species [3,9,
28]. We observed enhanced absorption of the alpha
band at 560 nm, a signature of the hexa-coordinated
form.
The far-ultraviolet circular dichroism spectrum (190–
250 nm) of ferric Ngb was typical of the globin family
(Fig. 1) showing mainly an alpha helical secondary
structure, in agreement with the X-ray structure [6].
The spectra for native Ngb had negative bands at 208
and 222 nm (Fig. 1), as expected for a high percentage
of alpha helix. Analysis of the secondary structure of
Ngb gave 78% alpha helix and 22% of other forms,
similar to HbA which was used as a control. The spec-
trum for Cygb showed slightly less alpha helix, as
expected if the extra residues (20 at each extremity)
are not helical.
Human Ngb has cysteine residues at positions 46
(CD7), 55 (D5) and 120 (G19). The cysteines CD7 and
D5 (Fig. 2), may form a disulfide bond within the CD
loop [16]. However, in mouse Ngb there are only two
cysteine residues (D5 and G19) and thus no intradisul-
fide bond is present. A similar circular dichroism spec-
trum was observed for mutant Ngb without cysteine
residues (triple mutation C46G C55S C120S, which we
refer to as CCC fiGSS), and for the mutant with
modified distal (E7) residue (data not shown). These
experiments suggest that the wild-type and mutant Ngb
proteins are correctly folded to the structure typical of
globins.
Thermal denaturation
Changes in the far-UV circular dichroism signal at
222.6 nm were used to follow the thermal unfolding.
The circular dichroism spectra vs. temperature revealed
a high thermal stability for Ngb and Cygb. The melt-
ing profiles are shown in Figs 3–6.
The melting temperature (T
m
) for human Ngb was
100 C for the ferrous form, 20 C higher than that
for horse heart myoglobin (Mb). The mutant of
Fig. 1. Circular dichroism spectra in the far-UV region of human
Ngb (…), human Cygb (——), and human HbA (– – –) at 25 Cin
1m
Mphosphate buffer at pH 7.
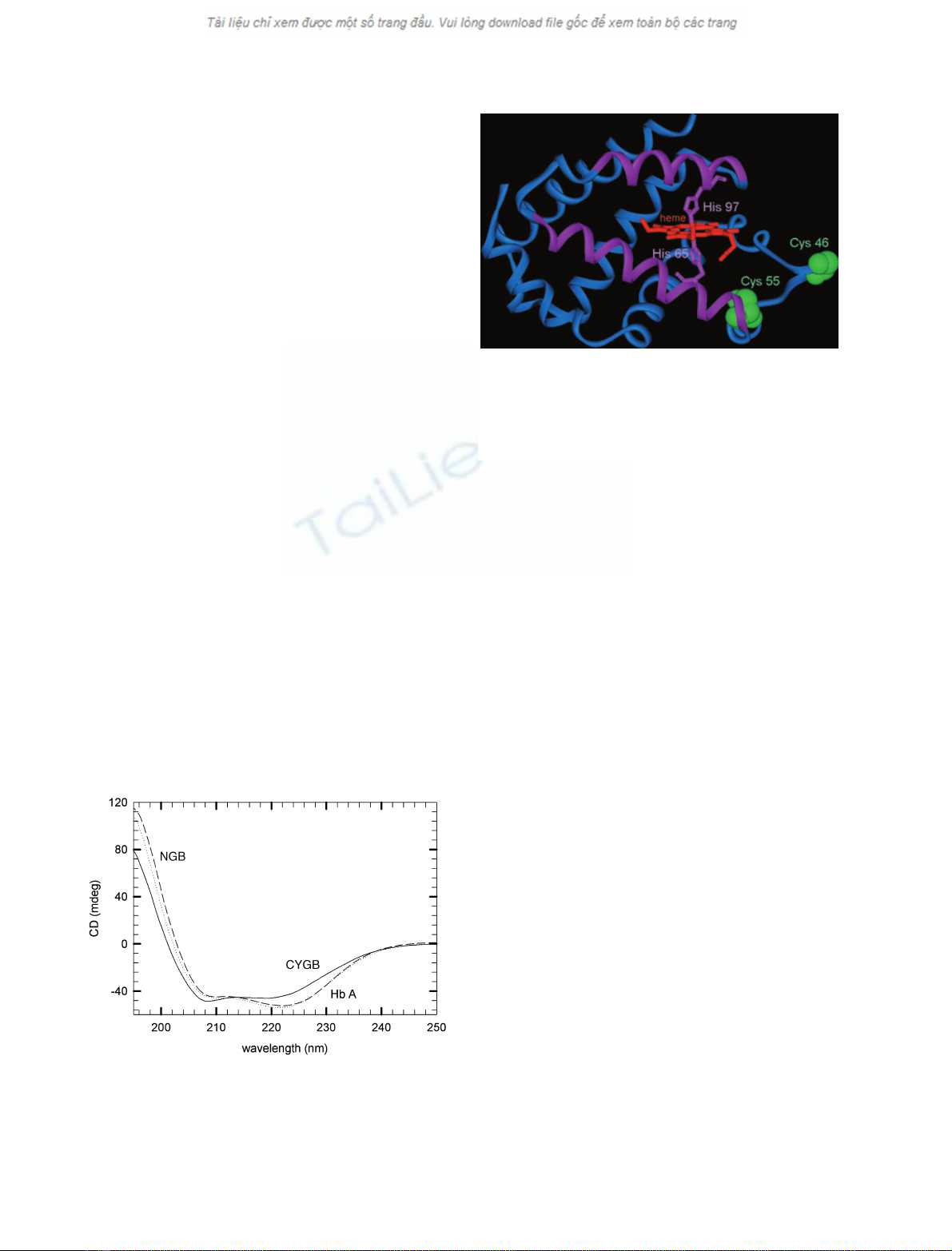
Fig. 2. Crystallographic structure of human Ngb mutant CCC fi
GSS (6). The hexa-coordination by the E7 (65) and F8 (97) histidines
helps stabilize the protein. The sites for the cysteines (CD7 and D5)
are shown in green; the disulfide bond (which decreases the E7
histidine affinity) decreases the melting temperature slightly, indica-
ting an indirect effect on the stability.
D. Hamdane et al. Thermal stability of neuroglobin
FEBS Journal 272 (2005) 2076–2084 ª2005 FEBS 2077

human Ngb without cysteines (CCC fiGSS) or sam-
ples of Ngb with dithiothreitol (to break the disulfide
bond, Fig. 3) or mouse Ngb (which does not have the
internal disulfide bond) had a T
m
value > 100 C
(Table 1). This would suggest that Ngb without the di-
sulfide bond is the most stable form. Because loss of
the disulfide bond in human Ngb increases the affinity
of the distal histidine (Table 1), the protein stability
may depend more directly on the hexa-coordination
rather than the disulfide bond.
The state of the iron atom may also influence T
m
(Fig. 4). For all species studied, we observed that the
deoxy form was the most stable (Table 1). The T
m
value of the deoxy ferrous species was obtained after
incubation of protein in dithionite under nitrogen.
Note that a rapid autoxidation at high temperatures
may prevent measurements on samples that remain
fully ferrous. The fact that ligands CO or CN
–
decrease the T
m
also suggests that the most stable form
is that in which the protein forms a sort of clamp
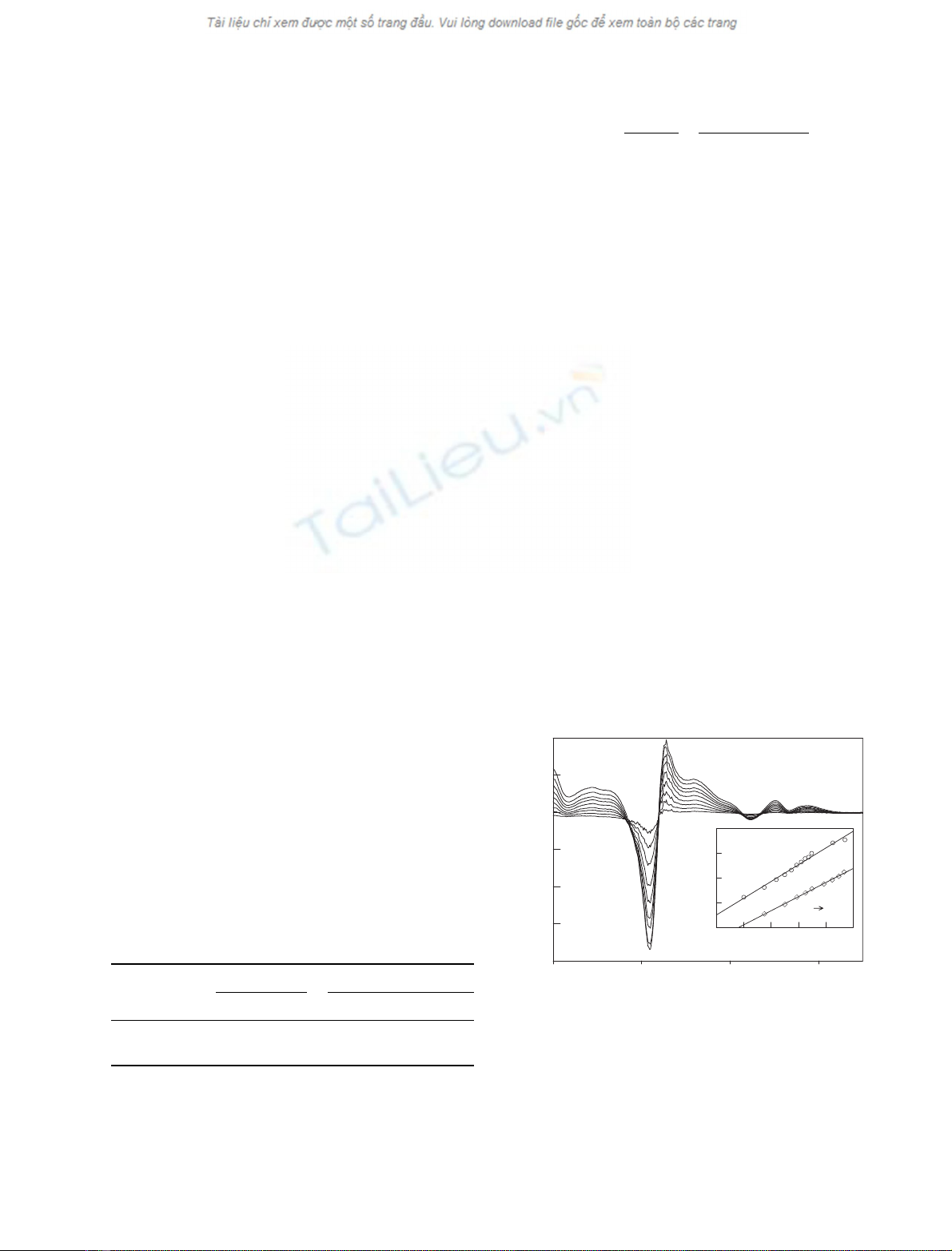
Fig. 3. Effect of the cysteine bridge of human Ngb on the thermal
stability. The melting temperature, corresponding to the peak of
this curve of the first derivative of the circular dichroism signal vs.
temperature, is shifted to higher values when the disulfide bond is
broken with dithiothreitol or for the mutant without cysteines.
Experiments were performed in 10 mMphosphate at pH 7 for ferric
human Ngb (d), Ngb with 0.5 mMdithiothreitol under nitrogen (j),
and the ferric mutant (CCC fiGSS) without cysteines (– –).
mouse Ngb
Temperature (°C)
80 85 90 95 100 105
fu
0.0
0.2
0.4
0.6
0.8
1.0
ferrous
ferrous
CO
ferric-CN ferric
Fig. 4. Melting profiles (fraction unfolded f
U
vs. temperature) of
mouse Ngb for different ligation states. Experimental conditions
were 1 mMphosphate buffer at pH 7 (at 25 C). Smooth curves are
simulations for a two state transition, as described in Experimental
procedures.
Temperature (°C)
60 70 80 90 100
fU
0.0
0.2
0.4
0.6
0.8
1.0
Cygb Ngb
Ngb
CCC->GSS
Mb
Drosophila
Fig. 5. Melting profiles of ferric hexa-coordinated globins. The frac-
tion unfolded f
U
vs. temperature is shown for Drosophila Hb (r),
Mb (d), human Ngb (m), the mutant CCC fiGSS of human Ngb
(.) and Mb (d). Experimental conditions were 1 mMphosphate
buffer at pH 7 (at 25 C).
[Guanidinium-chloride] (M)
0.0 0.5 1.0 1.5 2.0 2.5
Tm (°C)
60
70
80
90
100
110
120
wt CCC->GSS
40 60 80 100
0.0
0.2
0.4
0.6
0.8
1.0
temperature (°C)
f
U
2M 1M
0.5M
human Ngb
Fig. 6. Dependence of the melting temperature T
m
on [guanidinium
chloride] for wt human Ngb and the triple mutant CCC fiGSS. The
T
m
(Table 1) was obtained by extrapolation to 0 Mof guanidinium
chloride. The insert shows the thermal unfolding curve of human
Ngb at three concentrations of guanidinium chloride, in 10 mM
phosphate at pH 7.
Thermal stability of neuroglobin D. Hamdane et al.
2078 FEBS Journal 272 (2005) 2076–2084 ª2005 FEBS

around the heme group via the bis-histidyl binding to
the heme group. Furthermore, the decrease in T
m
upon
binding the external ligand could be underestimated at
high temperature due to oxidation or loss of the exter-
nal ligand.
Cygb and the globin from Drosophila are also hexa-
coordinated [2,3,28] and show various degrees of
enhanced stability (Fig. 5, Table 1). Cygb has an affin-
ity for the distal histidine 2.5-fold lower than human
Ngb and exhibits a T
m
5C lower than human Ngb.
A similar, but larger, effect was observed for the glo-
bin of Drosophila, in which the affinity of the distal
histidine is 14 times lower and the T
m
is decreased by
24 C relative to human Ngb. The very high stability
requires the hexa-coordinated state; for these cases the
T
m
may exceed 100 C, and additional curves were
measured at different concentrations of guanidinium
chloride (Fig. 6) to better determine the T
m
value.
Replacement of the distal histidine by valine, leucine
or glutamine in mouse Ngb leads to a loss of the
enhanced alpha absorption band in the deoxy form,
characteristic of the internal residue coordination (data
not shown). Relative to wild-type mouse Ngb, the sin-
gle mutation E7L in mouse Ngb caused a decrease of
20 C in thermostability, again suggesting a critical role
for His E7 in the enhanced thermal stability of Ngb.
Certain mutations of the distal histidine in Mb and Hb
lead to instability linked to a higher autoxidation rate
and ⁄or heme loss. Note that the E7 mutants are stable
with regard to O
2
binding, indicating that the mutation
does not affect the pocket to a large extent.
Reversibility
Although the thermal denaturation was irreversible for
human Hb, we observed a significant thermal reversi-
bility for mouse and human Ngb, and human Cygb.
The loss in helical content was 15%, estimated by the
difference at 222 nm between the initial and final circu-
lar dichroism spectra at 25 C after the temperature
cycle to 100 C (data not shown). The reversibility was
also tested by the absorption spectra (Fig. 7) and by
flash photolysis kinetics (Fig. 8). Ngb maintains a high
Table 1. Melting temperature (T
m
) and histidine affinity (K
His
¼
k
on
⁄k
off
) for hexa-coordinated globins.
T
m
(C) K
His
K
CN–,Mb
⁄K
CN–
Disulfide bond
Human Ngb (yes) 100 280
Human Ngb + dithiothreitol (no) 103 3300
Human Ngb CCC fiGSS (no) 103 4500
Ferric human Ngb (yes) 97 45
Ferric human Ngb CCC fiGSS (no) 101 428
Iron state
Ferrous mouse Ngb CO 95
Ferric mouse Ngb CN
–
94
Ferrous mouse Ngb (His) 103 2000
Ferric mouse Ngb (His) 100 137
Variable (E7) His affinity
Human Ngb CCC fiGSS 103 4500
Human Ngb (with disulfide bond) 100 280
Human Cygb 95 110
Drosophila Hb 76 18
Mouse Ngb His (E7) fiLeu 80 _
Horse heart Mb 81 < < 1
Human HbCO 71 < < 1
Fig. 7. Absorption spectra of ferrous human Ngb with dithiothreitol
(to break the disulfide bridge) at 25 C (solid line), after 5 min incu-
bated at 90 C, and finally at 25 C after the temperature cycle (s).
The spectrum for ferric human Ngb (with Soret band at 413 nm) is
also shown.
time (sec)
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
0.1
1
∆
A
N
human Ngb-CO
25°C
50°C
70°C
90°C
Fig. 8. Ligand rebinding kinetics for human Ngb at temperatures
from 25 to 90 C for samples equilibrated under 0.1 atm (100 lM)
CO, in 100 mMphosphate buffer at pH 7.
D. Hamdane et al. Thermal stability of neuroglobin
FEBS Journal 272 (2005) 2076–2084 ª2005 FEBS 2079
percentage (85%) of its initial characteristics after
the temperature cycle, whereas Mb shows 70%; the
fraction of functional HbA after the temperature cycle
was only 20%.
The shape of thermal denaturation curves of the
various globins may differ, suggesting different mecha-
nisms or degrees of cooperativity for the unfolding
transition. Classical denaturation between two states
results a cooperative denaturation with a maximum
slope at T
m
. The enthalpy of denaturation DH
m
of
Ngb and horse heart Mb was 72 and 110 kcalÆmol
)1
,
respectively. Cytoglobin and the globin of Drosophila
have lower values of DH
m
, 60 and 53 kcalÆmol
)1
,
respectively. Note that human Hb and Cygb are tetra-
meric and dimeric, respectively, and may involve a
more complicated mechanism including subunit disso-
ciation.
Ligand-binding kinetics
The circular dichroism spectra show that the protein is
still correctly folded at elevated temperatures, but do
not provide much information about protein function.
We studied ligand binding using flash photolysis to see
whether Ngb was functional at extreme temperatures.
The kinetics after CO photodissociation showed a bi-
phasic curve. The rapid phase corresponds to compet-
itive CO and His E7 association, whereas the slower
phase is the replacement of the E7 His by CO.
The kinetics for human Ngb at different tempera-
tures, up to 90 C, are shown in Fig. 8. The kinetic
curves show a steady progression vs. temperature, indi-
cating that there is no major change in the basic ligand-
binding properties. The increase in temperature leads to
an increase in the amplitude of the slow phase, indica-
ting that higher temperatures favor His vs. CO rebind-
ing; that is, the histidine association rate (k
His,on
) has a
higher activation energy than that for CO (Table 2).
Competition with the internal histidine ligand
decreases the affinity for external ligands such as CO:
KCO;obs ¼KCO;penta
1þKHis
¼kCO;on=kCO;off
1þkHis;on=kHis;off
ð1Þ
From the kinetic curves vs. [CO], one can extract three
of the rate parameters; the CO off rate must be deter-
mined independently. Equilibrium studies allow an
independent measure of the shift in observed affinity
due to the histidine.
Cyanide affinity
The absorption difference spectrum in the visible
region of ferric Ngb and cyanide derivative are shown
Fig. 9. The maximum absorption of ferric Ngb occurs
at 413 nm (Fig. 7). Cyanide binding to ferric Ngb
leads to a red shift in the Soret band; peak absorption
is seen at 416 nm for the mutant Ngb without cyste-
ines, and 417 nm for species with the disulfide bond.
The fraction saturation was calculated from the spec-
tral difference, and the titration curve (Fig. 9 insert)
gives a linear Hill plot.
The affinity of cyanide for ferric Ngb was much
lower than for Mb (Table 1), indicating competition
by the distal histidine, as in the ferrous form. The
affinity for cyanide was higher for mutant forms with-
out the distal histidine. For human Ngb without cys-
teine residues, the CN
–
affinity was lower, suggesting a
higher affinity for the competing histidine, as observed
in the ferrous form. Based on the shift in the CN
–
affinity, one can estimate the histidine affinity for the
Table 2. Activation and binding energies for CO binding to human
Ngb.
Species
His (kcalÆmol
)1
) CO (kcalÆmol
)1
)
E
on
E
off
DEE
on
E
off
DEDE
obs
Human Ngb 11 24 13 5.5 10 4.5 )9.5
a
Horse heart Mb 7.5 16 8.5 8.5
a
A value of )10 (± 3) kcalÆmol
)1
was determined from equilibrium
studies. Experimental conditions were 100 mMphosphate buffer at
pH 7.0, in the presence of 5 mMdithiothreitol.
wavelength (nm)
300 400 500 600
∆A
-0.20
-0.15
-0.10
-0.05
0.00
0.05
0.10
LOG(KCN µM)
1.6 2.0 2.4 2.8 3.2 3.6
LOG (Y/1-Y)
-2
-1
0
1
2
human Ngb
CCC GSS
428 nm
409 nm
wt
Fig. 9. Absorption difference spectra at various cyanide concentra-
tions, relative to the ferric Ngb form (without cyanide). The spectra
were measured at room temperature in 100 mMpotassium phos-
phate at pH 8. The insert shows the Hill plot of cyanide binding to
ferric Ngb. The shift to a lower CN
–
affinity for Ngb without the
disulfide bond is similar to that for oxygen in the ferrous form.
Thermal stability of neuroglobin D. Hamdane et al.
2080 FEBS Journal 272 (2005) 2076–2084 ª2005 FEBS


























