
Saposin B mobilizes lipids from cholesterol-poor and
bis(monoacylglycero)phosphate-rich membranes
at acidic pH
Unglycosylated patient variant saposin B lacks lipid-extraction
capacity
Natascha Remmel, Silvia Locatelli-Hoops, Bernadette Breiden, Guenter Schwarzmann and
Konrad Sandhoff
LIMES, Membrane Biology & Lipid Biochemistry Unit, c ⁄o Kekule
´-Institut fu
¨r Organische Chemie und Biochemie, University of Bonn,
Germany
Keywords
glycosphingolipid; lipid-binding protein;
lysosome; Sap; saposin
Correspondence
K. Sandhoff, LIMES, Membrane
Biology & Lipid Biochemistry Unit,
c⁄o Kekule
´-Institut fu
¨r Organische Chemie
und Biochemie, Gerhard-Domagk-Strasse 1,
53121 Bonn, Germany
Fax: +49 228 737 778
Tel: +49 228 735 346
E-mail: sandhoff@uni-bonn.de
(Received 8 February 2007, revised 26 April
2007, accepted 8 May 2007)
doi:10.1111/j.1742-4658.2007.05873.x
Sphingolipid activator proteins (SAPs), GM2 activator protein (GM2AP)
and saposins (Saps) A–D are small, enzymatically inactive glycoproteins of
the lysosome. Despite of their sequence homology, these lipid-binding and
-transfer proteins show different specificities and varying modes of action.
Water-soluble SAPs facilitate the degradation of membrane-bound glyco-
sphingolipids with short oligosaccharide chains by exohydrolases at the
membrane–water interface. There is strong evidence that degradation of
endocytosed components of the cell membrane takes place at intraendo-
somal and intralysosomal membranes. The inner membranes of the lyso-
some differ from the limiting membrane of the organelle in some typical
ways: the inner vesicular membranes lack a protecting glycocalix, and they
are almost free of cholesterol, but rich in bis(monoacylglycero)phosphate
(BMP), the anionic marker lipid of lysosomes. In this study, we prepared
glycosylated Sap-B free of other Saps by taking advantage of the Pichia
pastoris expression system. We used immobilized liposomes as a model for
intralysosomal vesicular membranes to probe their interaction with rec-
ombinantly expressed Sap-B. We monitored this interaction using SPR
spectroscopy and an independent method based on the release of radioac-
tively labelled lipids from liposomal membranes. We show that, after initial
binding, Sap-B disturbs the membrane structure and mobilizes the lipids
from it. Lipid mobilization is dependent on an acidic pH and the presence
of anionic lipids, whereas cholesterol is able to stabilize the liposomes. We
also show for the first time that glycosylation of Sap-B is essential to
achieve its full lipid-extraction activity. Removal of the carbohydrate moi-
ety of Sap-B reduces its membrane-destabilizing quality. An unglycosylated
Sap-B variant, Asn215His, which causes a fatal sphingolipid storage
disease, lost the ability to extract membrane lipids at acidic pH in the pres-
ence of BMP.
Abbreviations
BMP, bis(monoacylglycero)phosphate; GM2AP, ganglioside GM2 activator protein; PtdCho, phosphatidylcholine; pSap, Sap precursor;
RU, response unit; SAP, sphingolipid activator protein; Sap, saposin.
FEBS Journal 274 (2007) 3405–3420 ª2007 The Authors Journal compilation ª2007 FEBS 3405

For degradation, plasma membrane components are
transported to the lysosomes by endocytic membrane
flow. According to our current hypothesis [1], lipids
and proteins from the plasma membrane reach the
lysosomal compartment either as components of intra-
endosomal membranes or as part of the limiting mem-
brane. The luminal leaflet of the lysosomal-limiting
membrane is protected by a glycocalix against attack
by membrane-degrading enzymes and sphingolipid
activator proteins (SAPs) [2,3]. This glycocalix is com-
posed of membrane proteins, which are highly N-gly-
cosylated with mostly indigestible polylactosamine
structures [2]. By contrast, intralysosomal vesicles are
destabilized by high curvature and decreasing lateral
pressure. The diameter of intralysosomal vesicles has
been determined in tissues from SAP-deficient patients
to be in the range 50–100 nm [4,5]. It has been shown
that maturation of intraendosomal and intralysosomal
vesicles is accompanied by cholesterol depletion [6],
enrichment of the anionic lipid bis(monoacylglyc-
ero)phosphate (BMP) [6,7] and progressive acidifica-
tion of the lysosol. BMP (erroneously also called
LBPA, lysobisphosphatidic acid) is absent from the
perimeter membrane. Together with SAPs, which
include saposin (Sap)-A to -D and the GM2 activator
protein (GM2AP), it stimulates enzymatic sphingolipid
degradation on the inner membranes of the acidic
compartments of the cell [1]. Glycosylated [8–10] and
deglycosylated [11,12] Sap-B, as well as GM2AP [9],
have been characterized by in vitro studies as lipid-
binding proteins when lipids were presented as
micelles. The activators also act as transfer proteins,
which solubilize liposome-bound lipids and glyco-
sphingolipids with short carbohydrate chains and
transfer them to acceptor liposomes [9,13,14], by infer-
ence to water-soluble hydrolases, or antigen-presenting
proteins of the CD1-type [15,16]. The four Saps, A to
D, are formed biosynthetically by proteolytic process-
ing of a common precursor, prosaposin. They are
structurally related and share a 3D folding motif [17–
22]. Inherited deficiency of the Sap precursor (pSap)
and consequently of all four Saps results in a fatal
infantile lipid-storage disease. Cells from affected
human patients [23–25] and prosaposin knockout mice
[26] are characterized by simultaneous storage of cera-
mide and of glycosphingolipids with short oligosaccha-
ride chains. This is accompanied by a dramatic
accumulation of intralysosomal membranes [27].
In vitro, glycosylated [28,29] and deglycosylated Sap-
B [11] or unglycosylated recombinant Sap-B purified
from Escherichia coli [21,30] stimulate the degrada-
tion of sulfatide by arylsulfatase A. Native, glycos-
ylated Sap-B also stimulates the degradation of
globotriaosylceramide and digalactosylceramide by
a-galactosidase A [28]. The isolated hereditary defect
of Sap-B leads to atypical forms of metachromatic leu-
kodystrophy [31–35] with accumulation of the sub-
strates mentioned above [36].
The crystal structure of unglycosylated Sap-B [21]
shows a shell-like homodimer, enclosing a large hydro-
phobic cavity. Based on the structure and on binding
studies with lipids presented as micelles, the following
hypothetical mechanism for the interaction of Sap-B
with membranes was proposed [21]. In the open
conformation, the dimer interacts directly with the
membrane and rearranges the lipid alkyl chains. After
extraction of a lipid substrate, it changes into a closed
conformation. Thus, Sap-B may function as a lipid-
binding [20] and transfer protein of broad specificity
[8,12,13]. Although unglycosylated Sap-B promotes
many of the known functions of Sap-B, for example,
stochiometric binding of sulfatides [11,12] and promo-
tion of sulfatide hydrolysis by arylsulfatase A in vitro
[11,30] and cell culture [30], it apparently does not pro-
mote all the functions of glycosylated Sap-B needed
in vivo. A patient with an inherited defect in the glyco-
sylation site of Sap-B and therefore producing ungly-
cosylated Sap-B showed sulfatide storage in tissues,
had a phenotype resembling metachromatic leukodys-
trophy and died at the age of 5 years [31]. Two further
patients with a defect in the glycosylation sequence
also suffered from fatal sulfatide-storage diseases
[34,35].
To search for the functional defect caused by the
missing glycosylation of Sap-B, we prepared both gly-
cosylated and unglycosylated Sap-B and studied their
functional properties by analysing their ability to
mobilze lipids from lysosomal membranes.
In this study, we show for the first time the rele-
vance of the carbohydrate moiety of Sap-B for its
capacity to mobilize lipids from lipid bilayers under
conditions that mimic the lysosomal situation for
sphingolipid degradation. Therefore, lipid-mobilizing
capacity was analysed as a function of membrane cho-
lesterol and BMP, and as a function of pH and ionic
strength. We investigated wild-type Sap-B and two
variant forms, which lack the glycosylation site and
are therefore unglycosylated. The first recombinant
variant Sap-B, rvSap-B N215H(Pat), was discovered
in a patient suffering from an untypical form of meta-
chromatic leukodystrophy, caused by a homoallelic
point mutation [31], in the other variant, rvSap-B
N215Q, asparagine was substituted by glutamine in
order to delete the glycosylation site while retaining
similar properties of the amino acid residue at this
position.
Saposin B mobilizes lipids N. Remmel et al.
3406 FEBS Journal 274 (2007) 3405–3420 ª2007 The Authors Journal compilation ª2007 FEBS

Human Sap-B and variant Sap-B free of contamin-
ation by other Saps were prepared using the Pichia
pastoris expression system, which has already been suc-
cessful in the expression of functional GM2AP [37]
and Sap-A [38,39]. Sap-B was overexpressed in a high-
density fermentation process, the variant forms in
shake flask cultures, followed by a simple, three-step
purification that allows yields in the range of several
milligrams per litre necessary for reproducible biophys-
ical studies. Mobilization studies were performed using
two independent methods. SPR allowed us to immobi-
lize liposomes reproducibly and reversibly to the sur-
face of a sensor chip, present them to Sap-B and
monitor the interaction in real time. In a second
approach, liposomes containing radiolabelled lipids
were bound to octylsepharose and the release of lipids
after incubation with Sap-B was quantified by measur-
ing eluted radioactivity.
Results
Sap-B preparation
Cloning of recombinant Sap-B and transformation
into P. pastoris strain GS115
DNA fragments of 300 bp, containing the sequence
of human Sap-B or its variant forms, were generated
from the template pSap–pBHE plasmid by PCR and
cloned into pPIC9K. Ligation into the pPIC9K vector
led to inframe fusion of the export signal and the
Sap-B sequence. Electroporation into P. pastoris strain
GS115 and selection resulted in approximately eight
clones that were checked for expression.
Expression (flask ⁄bioreactor) and purification
of recombinant Sap-B
Clones with the highest expression levels were chosen
for large-scale purification. Shake-flask expressions
were used to establish the purification protocol and
prepare variant forms of Sap-B. The following yields
of purified protein were typically obtained in minimal
methanol medium after incubation for 4 days:
rgSap-B, 12.3 mgÆL
)1
; variants of Sap-B: rvSap-B
N215H(Pat), 3,9 mgÆL
)1
and rvSap-B N215Q, 1,9 mgÆ
L
)1
. Varying the medium (pH 6.0 by addition of
100 mmsodium phosphate) or induction time did not
increase the yield or change the glycosylation pattern
of wild-type Sap-B.
Expression was scaled up to yield 160 mgÆL
)1
of
total wild-type Sap-B in a bioreactor. Maximum yield
was reached after 120 h of methanol induction. Gly-
cosylated and unglycosylated species were present in
the supernatant in comparable amounts, whereas only
traces of other proteins could be detected by silver
staining.
The supernatant was loaded onto a cation-exchange
column, and Sap-B eluted in at least six separate frac-
tions, the first containing only glycosylated protein
and the last predominantly unglycosylated protein;
fractions in between contained a mixture. Further
separation of different glycoforms by lectin-affinity
chromatography (Concanavalin A) was successful,
whereas reversed-phase chromatography in the pres-
ence of organic solvents led to protein preparations
with decreased solubility and a tendency to form
aggregates unsuitable for SPR studies.
In contrast to shake-flask cultures, the supernatant
of bioreactor expressions was highly concentrated. As
a result, Sap-B tended to aggregate. In order to obtain
oligomeres (dimers to tetramers) free of aggregates,
both glycosylated and unglycosylated wild-type Sap-B
was further purified by gel filtration. The samples were
referred to as recombinant glycosylated (rgSap-B) and
recombinant unglycosylated Sap-B (ruSap-B).
Recombinant variant Sap-B (rvSap-B) expressed in
shake-flask cultures was subjected to cation-exchange
chromatography, followed by affinity chromatography
(Ni-nitriloacetic acid), in which the pure protein eluted
at pH 4.0.
Characterization of the recombinant Sap-B
and variant forms
The purity and identity of the recombinant proteins,
rgSap-B and rvSap-B, obtained after affinity chroma-
tography (Ni-nitriloacetic acid and gel filtration) were
confirmed by SDS ⁄PAGE [40], followed by silver stain-
ing and western blotting. Sap-B preparations used for
further studies had a purity > 95%, as checked by gel
electrophoresis and MALDI-TOF MS. Glycosylation
of rgSap-B could be completely removed by PNGase F
digestion. The masses of the carbohydrate residues
released by PNGase F digestion corresponded to (Glc-
NAc)
2
Man
9)12
. The major species of the rgSap-B
samples used in the following experiments started with
additional N-terminal amino acids, VEF, introduced
by the cloning procedure, minor species with EAYVEF
or YVEF due to heterogenous cleavage of the signal
sequence by yeast proteases.
The activity of glycosylated and unglycosylated Sap-B
preparations was tested in a Sap-B-dependent arylsulfa-
tase A assay for the hydrolysis of radiolabelled sulfati-
des using micellar (Fig. 1A) [8] and liposomal assay
conditions (Fig. 1B). In the liposomal assay, rgSap-B
exhibited 38%, ruSap-B 63% and rvSap-B 61%,
N. Remmel et al. Saposin B mobilizes lipids
FEBS Journal 274 (2007) 3405–3420 ª2007 The Authors Journal compilation ª2007 FEBS 3407
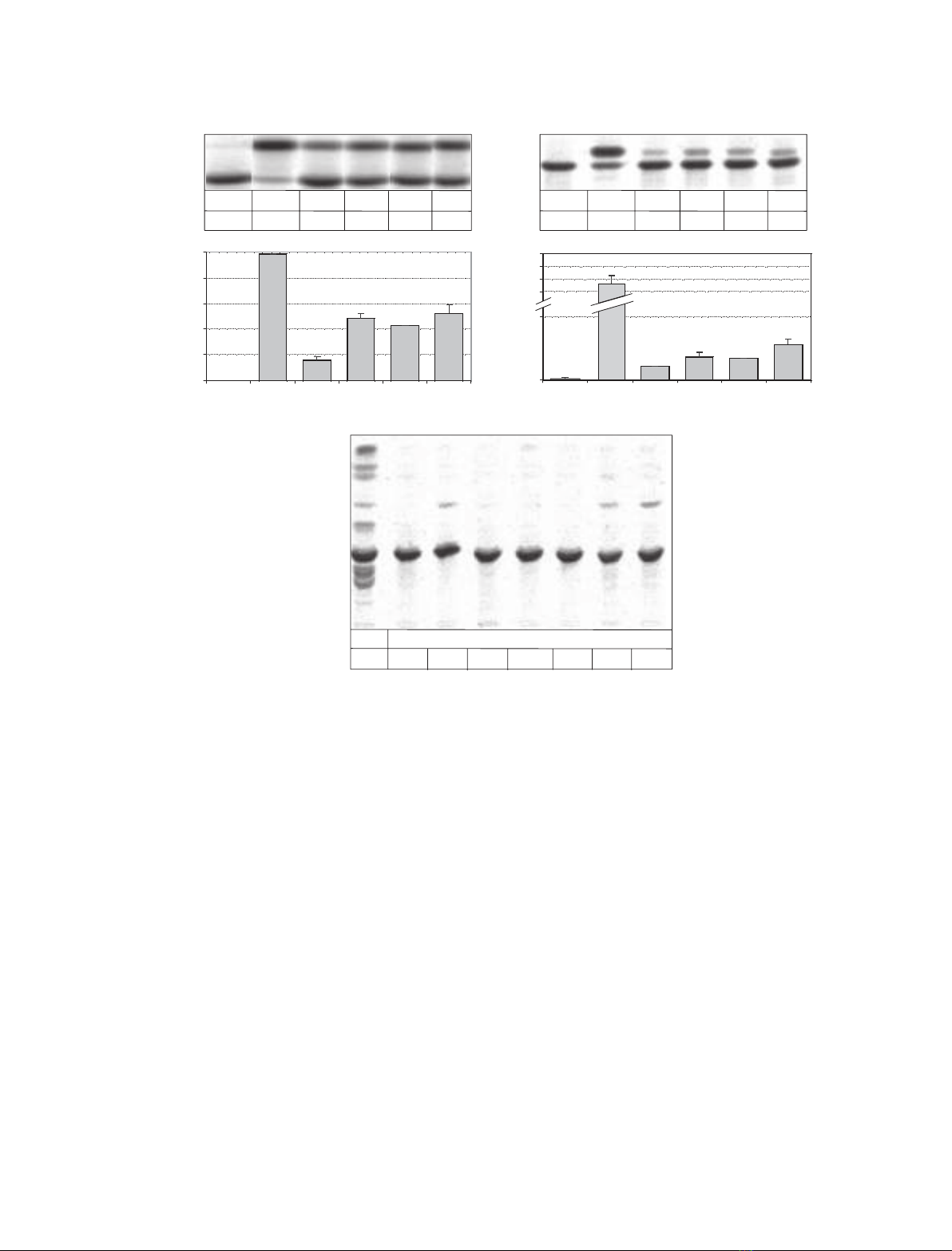
respectively, of the activity obtained for a Sap-B pre-
paration purified from Gaucher spleen.
After feeding to cultured cells deficient in Sap-A to
Sap-D rgSap-B exhibited 78% of the activity obtained
for Sap-B purified from human spleen whereas the
unglycosylated Sap-B (ruSap-B, rdSap-B N215D, and
rvSap-B N215Q) preparations showed no detectable
activity (Fig. 1C).
SPR
Basic experiments
To mimic the in vivo conditions of the protein–mem-
brane interaction at the surface of intraendosomal, and
intralysosomal vesicles, liposomes were presented to a
Sap-B solution. Defined measurement conditions and
sample preparation allowed monitoring the effect of
protein towards the lipid bilayer in the absence of
detergents or contaminating lipid-transfer proteins.
Unless otherwise indicated, the protein preparation
used for measurements was highly purified (> 95%)
recombinant glycosylated Sap-B, designated rgSap-B,
containing a C-terminal tetrahistidine tag. Liposomes
with an average diameter of 100 nm contained phos-
phatidylcholine (PtdCho), cholesterol and anionic
lipids (BMP, phosphatidic acid or sulfatide) in varying
proportions. The standard composition was defined as:
PtdCho, 61 mol%; BMP, 14 mol%; cholesterol,
AB
C
Sap-B
Sulf -
GalCer -
rgSap-B ruSap-B rvSap-B
N215Q
TDC +
__
_
____
hgSap-B
0
20
40
60
80
100
control TDC rgSap-B ruSap-B rvSap-B
N215Q
hgSap-B
Sap-B
Sulf -
GalCer -
rgSap-B ruSap-B rvSap-B
N215Q
TDC +
__
_
____
hgSap-B
0
10
control TDC rgSap-B ruSap-B rvSap-B
N215Q
hgSap-B
GalCer (% of sulfatide added)
GalCer (% of sulfatide added)
80
70
90
100
Sap-B
Sulf -
GalCer -
Cer -
rgSap-B rgSap-B
control pSap deficient
ruSap-BrdSap-B rvSap-B
N215Q
_
hgSap-B
_
Fig. 1. Determination of the Sap-B activity by hydrolysis of [
14
C]sulfatide in a micellar (A) and liposomal (B) in vitro assay, as well in an in vivo
assay in pSap-deficient fibroblast (C). In the in vitro assay (A, B) 0.5 nmol [
14
C]sulfatide, 30 mU arylsulfatase A, 0.33 nmol Sap-B or as a con-
trol 100 nmol taurodesoxycholate (TDC) were incubated for 2 h at pH 4.2 and 37 C. Liposomes with 10 mol% [
14
C]sulfatide, 10 mol% cho-
lesterol, and 80 mol% phosphatidylcholine were used in the liposomal assay (B). Lipids were separated by TLC and quantified. (C) Effect of
different Sap-B preparations on the degradation of radiolabelled sulfatide in pSap-deficient fibroblasts and healthy control fibroblasts. Cells
were preincubated with Sap-B (25 lgÆmL
)1
medium) and after 24 h they were fed with [
14
C]sulfatide (0.33 nmolÆmL
)1
medium). The isolated
lipids were separated by TLC. The data are the mean of a double determination with a deviation less than ± 10% of the mean. Cer, cera-
mide; GalCer, galactosylceramide; Sulf, sulfatide; TDC, taurodeoxycholate; d, deglycosylated; g, glycosylated; h, human; r, recombinant;
u, unglycosylated; v, variant.
Saposin B mobilizes lipids N. Remmel et al.
3408 FEBS Journal 274 (2007) 3405–3420 ª2007 The Authors Journal compilation ª2007 FEBS
25 mol%, and used in all control experiments. For
SPR measurements, liposomes were immobilized on a
PioneerL1 sensor chip (Biacore), presenting alkyl
chains of appropriate length (C
8–12
) that inserted into
the lipid bilayer. It has been shown by electron micros-
copy [41], fluorescence microscopy [42], or by analy-
sing the release of a fluorescent marker from liposomes
while loading the chip [43,44], that the sensor chip cap-
tures mostly intact liposomes. All SPR measurements
were performed at room temperature.
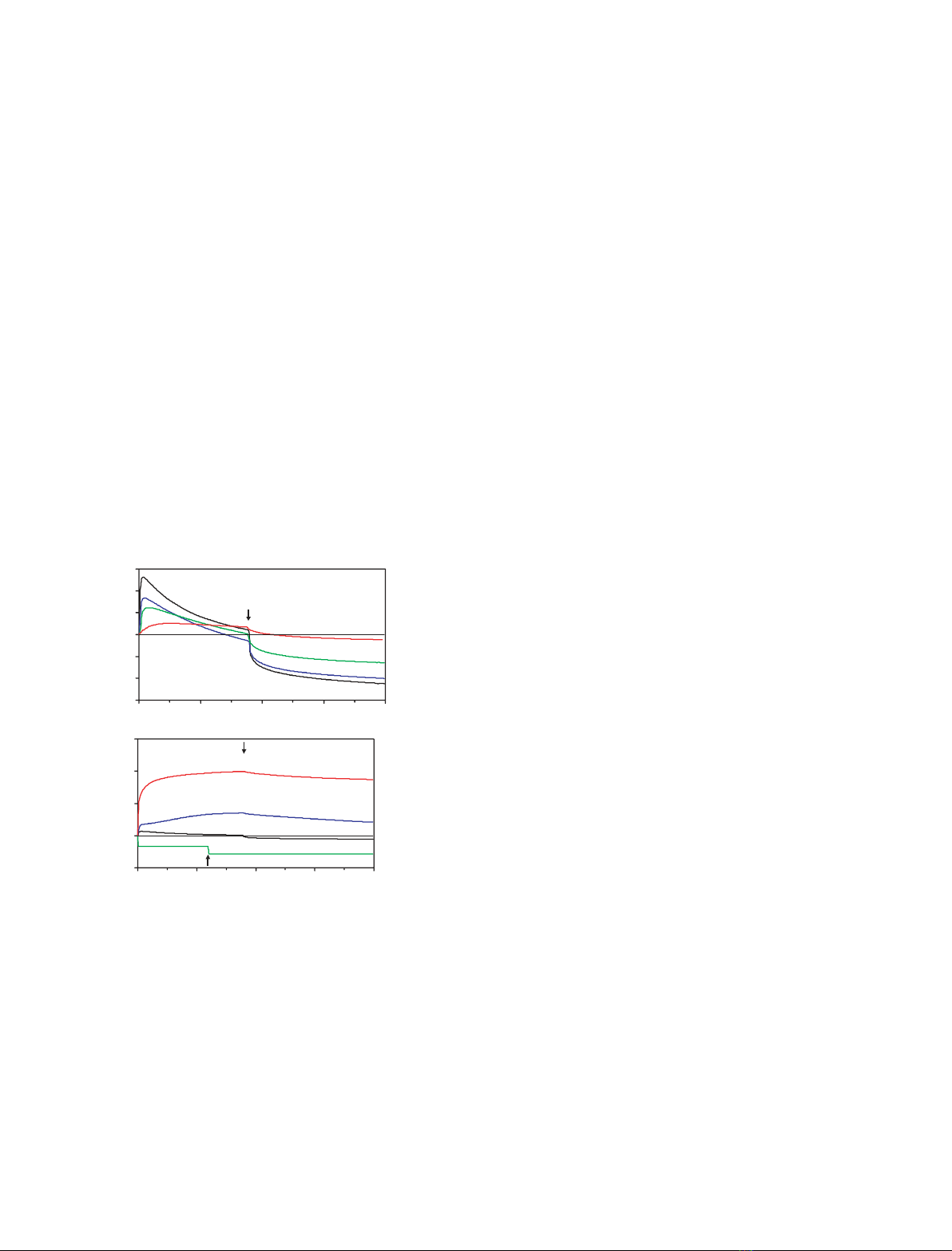
Interaction of control substances with immobilized
liposomes
When the running buffer (50 mmsodium citrate,
pH 4.2) was used without any protein addition as a
negative control, no alteration of the baseline (corres-
ponding to the x-axis) was observed. In contrast, the
detergent Chaps (20 mmin running buffer) led to an
instant decrease in the signal, far below the baseline
(Fig. 2B), reaching, after further washing with buffer
solution, a final response unit (RU) value that corres-
ponds to the signal obtained before immobilization of
liposomes (data not shown), indicating complete
removal of lipids from the chip and also of Chaps,
which might have been bound to the chip before the
washing period. In order to minimize the incubation
time of the detrimental detergent on the chip surface,
the association time was shortened to 120 s instead of
180 s for other samples. The decrease at 120 s was due
to the removal of Chaps from the flow cell. The con-
trol proteins BSA (5 lmin running buffer) and cyto-
chrome c(5 lmin running buffer), which are known
not to disturb membrane structures, rapidly associated
with the immobilized liposomes, reaching equilibrium.
The following dissociation after injection of pure run-
ning buffer was slow and incomplete in the measured
time interval (Fig. 2B). rgSap-B strongly bound to the
liposome-free chip surface. Complete removal could
not be achieved by buffer alone but required denatur-
ing agents such as SDS (data not shown).
rgSap-B mobilizes lipids from immobilized liposomes
Addition of rgSap-B (0.05–5.0 lm, in running buffer)
initially increased the signal, indicating protein binding
to immobilized liposomes. The signal reached a maxi-
mum value and then started to decrease steadily while
protein was still injected (Fig. 2A). Similar to the
Chaps curve (Fig. 2B), the signal dropped below the
baseline for rgSap-B concentrations of 1 lmor more,
suggesting release of lipids from the liposomes. This
process continued even more pronounced when protein
was exchanged for pure running buffer, as the loss of
material from the chip was no longer compensated for
by binding of Sap-B. The change from buffer contain-
ing Sap-B to pure buffer divides the interaction process
in association and dissociation phase, but does not
represent a new kind of interaction with kinetic, ther-
modynamic parameters, or physiological relevance, of
its own. Both binding and mobilization intensified in a
concentration-dependent manner, although the increase
from 1 to 5 lmrgSap-B did not accelerate the mobil-
ization process further, probably because the extra-
ction process was overlaid by binding of Sap-B to the
chip.
SPR measurements were very sensitive towards the
(unphysiological) treatment of protein samples with
organic solvents (data not shown). Consequently,
reversed-phase chromatography was not used in our
purification protocol.
Extraction of radiolabelled membrane lipids by Sap-B
As described above, SPR measurements using Chaps
or rgSap-B as analytes resulted in curves that reach
-1500
-1000
-500
0
500
1000
1500
0 100 200 300 400
time (s)
Response Units (RU)
rgSap-B
(µ
M
)
A
0.05
5.0
1.0
0.5
0.0
-10000
0
10000
20000
30000
0 100 200 300 400
time (s)
Response Units (RU)
B
rgSap-B (5 µM)
BSA (5 µM)
cyt c (5 µM)
CHAPS (20 mM)
Fig. 2. rgSap-B mobilizes lipids from BMP-containing liposomes.
Liposomes containing 61 mol% PtdCho, 25 mol% cholesterol and
14 mol% BMP were immobilized on the surface of a L1 chip (Bia-
core). Increasing concentrations of rgSap-B were injected into the
flow cell under acidic conditions (50 mMsodium citrate, pH 4.2) at
a flow rate of 20 lLÆmin
)1
for 180 s during the association phase.
This was followed by the injection of protein free buffer (dissoci-
ation phase), indicated by an arrow. Representative binding curves
are shown in (A). Curves falling below the baseline suggest mobil-
ization of membrane lipids. Under the same conditions the deter-
gent Chaps removed all bound membrane lipids from the sensor
chip, whereas cytochrome cand BSA bound to the membrane
without removing lipids (B).
N. Remmel et al. Saposin B mobilizes lipids
FEBS Journal 274 (2007) 3405–3420 ª2007 The Authors Journal compilation ª2007 FEBS 3409



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





