RESEARCH ARTICLE Open Access
Treatment of alcohol dependence with low-dose
topiramate: an open-label controlled study
Thomas Paparrigopoulos
1*
, Elias Tzavellas
1†
, Dimitris Karaiskos
1†
, Georgia Kourlaba
2†
, Ioannis Liappas
1†
Abstract
Background: GABAergic anticonvulsants have been recommended for the treatment of alcohol dependence and
the prevention of relapse. Several studies have demonstrated topiramate’s efficacy in improving drinking behaviour
and maintaining abstinence. The objective of the present open-label controlled study was to assess efficacy and
tolerability of low-dose topiramate as adjunctive treatment in alcohol dependence during the immediate post-
detoxification period and during a 16-week follow-up period after alcohol withdrawal.
Methods: Following a 7-10 day inpatient alcohol detoxification protocol, 90 patients were assigned to receive
either topiramate (up to 75 mg per day) in addition to psychotherapeutic treatment (n = 30) or psychotherapy
alone (n = 60). Symptoms of depression and anxiety, as well as craving, were monitored for 4-6 weeks immediately
following detoxification on an inpatient basis. Thereafter, both groups were followed as outpatients at a weekly
basis for another 4 months in order to monitor their course and abstinence from alcohol.
Results: A marked improvement in depressive (p < 0.01), anxiety (p < 0.01), and obsessive-compulsive drinking
symptoms (p < 0.01) was observed over the consecutive assessments in both study groups. However, individuals
on topiramate fared better than controls (p < 0.01) during inpatient treatment. Moreover, during the 4-month
follow up period, relapse rate was lower among patients who received topiramate (66.7%) compared to those who
received no adjunctive treatment (85.5%), (p = 0.043). Time to relapse in the topiramate augmentation group was
significantly longer compared to the control group (log rank test, p = 0.008). Thus, median duration of abstinence
was 4 weeks for the non-medicated group whereas it reached 10 weeks for the topiramate group. No serious side
effects of topiramate were recorded throughout the study.
Conclusions: Low-dose topiramate as an adjunct to psychotherapeutic treatment is well tolerated and effective in
reducing alcohol craving, as well as symptoms of depression and anxiety, present during the early phase of alcohol
withdrawal. Furthermore, topiramate considerably helps to abstain from drinking during the first 16-week post-
detoxification period.
Background
Alcohol dependence is a multifactorial disorder influ-
enced by interacting genetic, biological, psychological
and environmental factors. Pharmacologically, alcohol
is considered to be a potent central nervous system
depressant and its action is mediated through multiple
neurotransmitter systems, including the GABAergic, glu-
tamatergic, dopaminergic serotoninergic, and opiatergic
system. This complex neurobiological network, which is
involved in the regulation of alcohol preference, intake,
and the rewarding and craving components of alcohol
dependence, has been the target of various pharmacolo-
gical agents, albeit with only limited success. In this
context, there has been a growing interest in the use of
anticonvulsant medications in the field because these
agents may act on the neurobiological substrate of
addiction [1,2]. In this vein, the anticonvulsant topira-
mate has been suggested to be promising for treating
alcohol dependence [3,4]. Although the mechanism is
unclear, modulation of the dopamine reward pathways
of the brain through antagonizing excitatory glutamate
receptors at a-amino-3-hydroxy-5-methylisoxazole-4-
propionic acid and kainate receptors and inhibiting
* Correspondence: tpaparrig@med.uoa.gr
†Contributed equally
1
Athens University Medical School, 1st Department of Psychiatry, Eginition
Hospital, Athens, Greece
Full list of author information is available at the end of the article
Paparrigopoulos et al.BMC Psychiatry 2011, 11:41
http://www.biomedcentral.com/1471-244X/11/41
© 2011 Paparrigopoulos et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative
Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is properly cited.

dopamine release [5,6] within the mesocorticolimbic sys-
tem while enhancing inhibitory GABA (by binding to a
site of the GABA-A receptor) [7], has been proposed as
being responsible for its effectiveness [8]. This dual
action of topiramate is supposed first to lead to a dopa-
mine decrease in the nucleus accumbens in response to
alcohol ingestion, and consequently to a reduction of its
rewarding/reinforcing potential, and second to minimize
withdrawal symptoms by moderating the effect of
chronic alcohol consumption on neural system excitabil-
ity. Thus, targeting at both facets of addiction, i.e., crav-
ing and feelings of inner tension and discomfort, relapse
may be less likely. However, several issues regarding
dosing, duration and tolerability of treatment with topir-
amate have not been adequately addressed as yet.
Treatment of alcohol dependence is a two-phase pro-
cess, which aims at alcohol withdrawal and subsequent
long-term abstinence and relapse prevention [9]. Depend-
ing on the phase, different priorities may be set. Thus,
pharmacotherapy during and immediately after detoxifica-
tion may protect from withdrawal dysphoric symptoms
and can reduce anxiety and symptoms of depression
[10-12]. Thereafter, medication can be used to reduce
craving and reward from alcohol use. GABAergic and
glutamatergic medications might be promising candidates
for helping during both phases of alcohol dependence
treatment [13].
The objective of the present open-label controlled study
was to assess the efficacy and tolerability profile of low-
dose topiramate as adjunctive treatment in alcohol depen-
dence during the immediate post-detoxification period
and during a 16-week follow-up period after alcohol with-
drawal. The choice of low-dose topiramate was made
based on the existing, albeit limited, literature, which sug-
gests that even low doses of this medication can be benefi-
cial in preventing alcohol relapse [14,15,4]; moreover, a
less aggressive approach with milder side-effects could be
advantageous in terms of treatment adherence. A control
non-medicated group of alcohol-dependent individuals
was used for comparisons in terms of anxiety and depres-
sive symptoms, craving and drinking outcome.
Methods
Study design - participants
The study was an open-label controlled clinical trial. Par-
ticipants were assigned either to a standard alcohol
detoxification group (see below) or to a topiramate aug-
mentation group. Assignment to the topiramate augmen-
tation group was made on a 2:1 ratio; thus, every third
intake was assigned to the topiramate group. In total, 90
alcohol-dependent individuals who consecutively con-
tacted the Drug and Alcohol Addiction Clinic of the
Athens University Psychiatric Clinic at the Eginition
Hospital in Athens, Greece, were enrolled in the study.
Patients had to fulfil the DSM-IV-TR [16] diagnostic cri-
teria for alcohol abuse/dependence and were admitted
for inpatient alcohol detoxification. Informed consent
was obtained from the participants after providing
detailed information on the objectives of the study and
the research/therapeutic protocol. All procedures were
approved by the ethical committee of our institution.
(“Committee on Medical Ethics of the Eginition Hospital”
& Reference No: 1178).
The inclusion criteria were: a) age 18-65 years,
b) absence of a serious physical illness (as assessed
through physical examination and routine laboratory
screening), c) absence of another pre- or co-existing
major psychiatric disorder on the DSM-IV-TR axis I,
d) absence of another drug abuse, and e) participants
with affective or anxiety symptoms were not excluded
from the study if concurrent with an alcohol-abusing
period; individuals who fulfilled a DSM-IV-TR diagnosis
of depressive or anxiety disorder (assessed through the
Schedules for Clinical Assessment in Neuropsychiatry
[SCAN] [17] and information obtained from a close
relative) were excluded from the study if relevant cri-
teria were met prior to the onset of alcoholism or dur-
ing periods of abstinence.
Participants were assigned to two study groups: the
control group (n = 60), which included subjects treated
with a standard alcohol detoxification protocol, and
the topiramate augmentation group (n = 30), which
included patients who were additionally given topira-
mate (up to 75 mg/day in 2 divided doses). Topiramate
was initiated at a daily dose of 25 mg, before stopping
the last dose of 5 mg diazepam, and was gradually
increased up to 75 mg/day over three weeks (mean
dose: 55.0 ± 19.03 mg/day).
The standard alcohol detoxification protocol was
initiated and completed one week (7-10 days) after
admission to the ward. This protocol includes vitamin
replacement (vitamins C, E and B complex) and oral
administration of diazepam (30-60 mg in divided doses),
with gradual taper off over a week. Thereafter, both
groups were given a standard treatment program with
cognitive-behavioural short-term psychotherapy of 4-
6 week duration (i.e. during their inpatient treatment).
After discharge, patients were assessed at a weekly basis
for 4 more months in order to monitor their course and
abstinence from alcohol. Assessment of abstinence from
alcohol was based on self reports, but it was further
cross-checked with a family member to ascertain accu-
racy of information. Also, serum g-glutamyl transpepti-
dase (g-GT) and an alcohol breath test at each visit were
used to control abstinence. No discrepancies were
observed between these measures of abstinence through-
out the study. Although there is ongoing debate regard-
ing the reliability of self-reports of alcohol consumption,
Paparrigopoulos et al.BMC Psychiatry 2011, 11:41
http://www.biomedcentral.com/1471-244X/11/41
Page 2 of 7

it has been shown that data thus collected are a valid
source of information in the case of dependent indivi-
duals [18]. Furthermore, g-GT is considered to be a reli-
able marker of alcohol relapse detection [19].
From the total sample (n = 90), eighty-five subjects
(n = 85) were included in the final statistical analysis
because five participants from the control group had
occasionally used benzodiazepines during the follow-up
period on their own initiative, and this was considered
as protocol violation.
Measures
Participants were diagnosed by the Schedules for Clinical
Assessment in Neuropsychiatry [SCAN] and assessed
through the Composite International Diagnostic Interview
[20] (CIDI; section on alcohol consumption) for their pat-
tern of alcohol abuse, potential major life problems related
to alcohol consumption and the occurrence of withdrawal
symptoms in the past. All data pertaining to alcohol use
were self-reported but in order to ascertain accuracy of
information a relative was also interviewed to corroborate
current status and psychiatric history. Furthermore, socio-
demographic data (age, socioeconomic status, marital sta-
tus, level of education) and previous psychiatric history
(pre-existent diagnosis, medication, number of hospitaliza-
tions) were recorded. Symptoms of depression and anxiety
were assessed with the Hamilton Depression Rating Scale
(HDRS) [21] and the Hamilton Anxiety Rating Scale
(HARS) [22]. Obsessive thoughts about alcohol use and
compulsive behaviours toward drinking (facets of craving)
were estimated with the Obsessive Compulsive Drinking
Scale (OCDS) [23]. Overall functioning was assessed using
the Global Assessment Scale (GAS) [24]. The severity of
withdrawal symptoms was evaluated twice daily during the
first week of alcohol withdrawal with a modified version of
the Addiction Research Foundation Clinical Institute
Withdrawal Assessment for Alcohol (CIWA-Ar) [25].
Adverse effects of treatment in both groups were moni-
tored through an adapted version of the Systematic
Assessment for Treatment Emergent Events (COMBINE
SAFTEE) scale, which is a structured instrument for col-
lecting adverse events adapted for clinical studies in the
alcoholism field [26]. Assessments were done at three time
points; initially within 48 h upon entering the program
(time point 0) and subsequently at 21 ± 2 day intervals
(time point 1 & 2) over the 4-6 week study period.
Statistical analysis
The number of subjects that entered into the final analysis
was eighty-five (n = 85). Independent samples t-tests were
used to evaluate differences between groups in terms of
symptoms of depression, anxiety, global functioning and
obsessive-compulsive drinking scores at the different time
points. Within groups differences were estimated with
repeated measures analysis of variance (RMANOVA). For
all reported values the means (± SD) were calculated. Chi-
square statistics were used to compare categorical vari-
ables, as appropriate. Cox proportional hazards model was
used to estimate the hazard ratio (HR) of achieving 16
weeks of continuous abstinence and the Kaplan-Meier
method was applied to calculate the cumulative probability
function of reaching 16 weeks of abstinence for the topira-
mate and the control group. The log-rank test was used to
compare the cumulative probability functions. The pro-
portional hazard assumption of Cox model was assessed
through the appropriate graph. All tests were two-tailed
with statistical significance set at p < 0.05. Data analysis
was performed using the SPSS statistical software package
(SPSS Inc. Chicago, IL, USA).
Results
No significant differences were observed between the
control and topiramate group in terms of their sociode-
mographic characteristics, as well as the variables related
to alcohol abuse history and withdrawal symptoms dur-
ing the first week of abstinence (Table 1). As regards
psychopathological symptoms both groups had similarly
high scores on the HDRS, HARS and OCDS, and low
GAS scores upon admission (time 0), which represent a
serious psychosocial impairment. A marked improve-
ment on all these measures was observed in the two
subsequent assessments (time 1 & time 2) in both study
groups [depression (p < 0.01), anxiety (p < 0.01), and
obsessive-compulsive drinking symptoms (p < 0.01)].
However, subjects on topiramate did significantly better
than controls concerning mood improvement, i.e., anxi-
ety and depression (p < 0.05), and craving as well (p <
0.01) (Table 2).
Table 1 Sociodemographic characteristics and variables
related to alcohol consumption of the sample (N = 85)
Topiramate
group
(N = 30)
Control group*
(N = 55)
Mean age, years (± SD) 43.8 ± 8.1 46.3 ± 11.0
Sex (M, F) M: 27, F: 3 M: 48, F: 7
Family status (S, M, D) S: 8, M: 16, D: 6 S: 10, M: 33, D: 12
Socioeconomic status (H, M, L) H: 1, M: 25, L: 4 H: 2, M: 39, L: 14
Educational years 7.6 ± 3.1 8.3 ± 3.8
Age at onset, years (± SD) 24.1 ± 6.2 27.3 ± 9.6
Mean alcohol consumption
(gr/day)
272 ± 115 284 ± 140
Mean (CIWA-Ar) during the first
week
26.1 ± 6.7 25.7 ± 6.3
M, Male; F, Female; S, Single; M, Married; D, Divorced/separated/widowed; H,
High; M, Middle; L, Low. CIWA-Ar, Clinical Institute Withdrawal Assessment for
Alcohol, Revised.
*All comparisons between groups were non-significant.
Paparrigopoulos et al.BMC Psychiatry 2011, 11:41
http://www.biomedcentral.com/1471-244X/11/41
Page 3 of 7
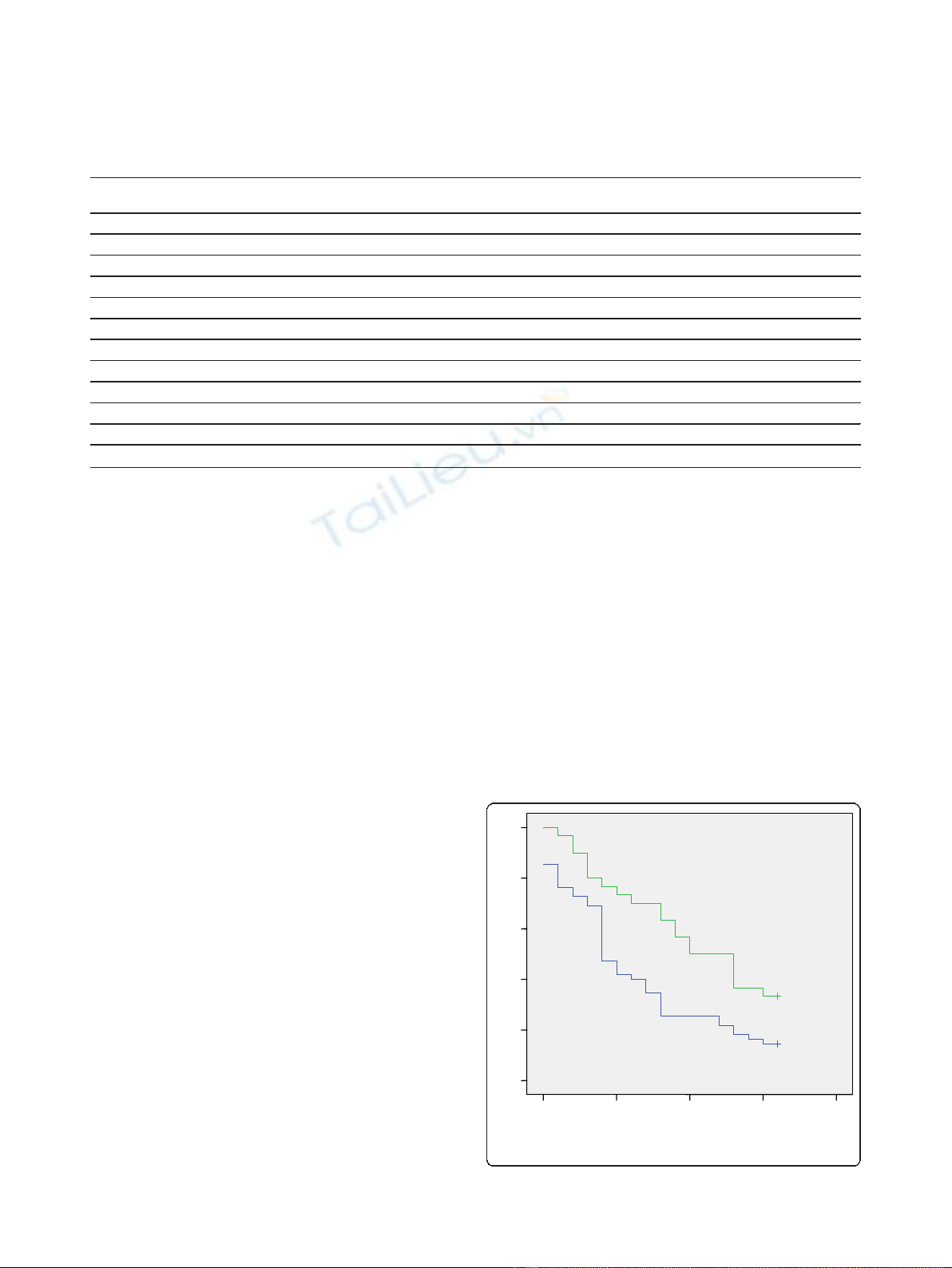
Long-term outcome in terms of abstinence from alco-
hol was better for the topiramate augmentation group.
Thus, although 67 patients in total (78.8%) had relapsed
to alcohol use by the end of the study (16 weeks after dis-
charge), relapse rate was significantly lower in the topira-
mate group (66.7%) compared with the control group
(85.5%) (p = 0.043). Also, median duration of abstinence
in the topiramate group was significantly longer com-
pared to the non-medicated group (10 weeks vs. 4 weeks;
log rank test, p = 0.008, Figure 1). Cox proportional
hazard model showed that risk of relapse was 56% lower
among patients receiving topiramate compared to con-
trols (HR = 0.515, 95% CI: 0.304 - 0.874, p = 0.014).
Reported adverse effects are presented on Table 3.
A considerable proportion (> 10%) of the topiramate aug-
mentation group had some adverse effects, but no significant
difference was recorded compared with the control group,
except for somnolence which was significantly more frequent
in the topiramate group (23.3% vs. 5.4%). All adverse effects
were tolerable and there were no dropouts from the study.
Discussion
The main finding of the present study is that low-dose
topiramate given as a treatment adjunct is well-accepted
and effective in reducing craving for alcohol and symp-
toms of anxiety and depression during the early phase
of alcohol withdrawal. Furthermore, topiramate com-
bined with a psychotherapeutic intervention improves
abstinence from drinking during the first 16-week post-
detoxification period, in comparison with alcohol-depen-
dent individuals receiving psychotherapy alone.
Although topiramate is not currently approved for the
treatment of alcohol dependence [27], several rando-
mized double-blind placebo-controlled trials have
demonstrated its efficacy in improving drinking beha-
viour and maintaining abstinence [28-30]. Compared to
the standard medications approved for alcohol depen-
dence, topiramate has been found to be inferior to disul-
firam in terms of days to relapse [31] and superior to
naltrexone in reducing craving [32] and improving some
other critical measures of drinking behaviour [30]. The
Table 2 Mean scores ± SD of the various measures of psychopathology and craving at the different time points of
assessment (time 0 ®time 2) in the control and the topiramate augmentation group
Variable
(Mean ± SD)
Group 1
st
Assessment (time 0) 2
nd
Assessment
(time 1)
3
rd
Assessment
(time 2)
HDRS
Controls 38.7 ± 7.6 15.9 ± 8.6+ 8.1 ± 6.6∞
Topiramate 39.6 ± 5.5 12.5 ± 3.1*+ 4.9 ± 3.1*∞
HARS
Controls 30.5 ± 10.2 13.9 ± 6.7+ 7.1 ± 6.2∞
Topiramate 31.8 ± 5.3 10.9 ± 3.6*+ 4.3 ± 3.8*∞
GAS
Controls 46.7 ± 5.1 75.6 ± 9.3+ 85.4 ± 8.5∞
Topiramate 46.6 ± 4.7 74.3 ± 6.7+ 84.3 ± 5.6∞
OCDS
Controls 37.2 ± 8.3 17.3 ± 3.9+ 13.3 ± 2.8∞
Topiramate 37.6 ± 7.8 15.3 ± 3.9*+ 10.3 ± 3.1**∞
Statistics: Between groups (independent samples t-test); within groups (RMANOVA).
*Significant difference between controls and topiramate augmentation group (< 0.05).
** Significant difference between controls and topiramate augmentation group (< 0.01).
+ Significant difference between 2
nd
and 1
st
assessment (< 0.01).
∞Significant difference between 3rd and 2nd assessment (< 0.01).
week of relapse
20
15
10
5
0
Cum Survival
1,0
0,8
0,6
0,4
0,2
0,0
Topiramate group
Control group
Figure 1 The cumulative probability function of reaching
16 weeks of abstinence by group.
Paparrigopoulos et al.BMC Psychiatry 2011, 11:41
http://www.biomedcentral.com/1471-244X/11/41
Page 4 of 7

precise mechanism of topiramate’s favourable action is
unclear. It may be that it modulates GABAergic trans-
mission in the central amygdala, a brain region impli-
cated in the regulation of emotionality and alcohol
intake [33,34]. Also, it has been shown that GABA
receptors undergo allosteric modulation by ethanol and
mediate the acute and chronic effects of alcohol, includ-
ing tolerance, dependence and withdrawal [35]. On the
other hand, topiramate enhances the inhibitory function
of GABA, antagonizes excitatory glutamate receptors,
and inhibits dopamine release [36].
Topiramate has been used for the treatment of alcohol
dependence in outpatient settings. Doses ranging from
150 to 300 mg/day have shown promising results, in terms
of significant improvement in several dependence-related
parameters [3]. However, a major concern has been topir-
amate’s adverse effects, which are prominent especially
during the titration period, appear to be dose-related but
usually subside with continued treatment [37,38]. Thus,
the majority of patients who discontinue topiramate treat-
ment, due to its side effects, do so early in treatment. In
this line of thought, a key objective of the present study
was to establish the efficacy and side effect profile of low-
dose topiramate (up to 75 mg/day) that might improve
adherence to treatment. In our sample, a considerable pro-
portion (> 10%) of the topiramate augmentation group
had some adverse effects, but no significant difference was
recorded compared with the control group. However,
these adverse effects were tolerable and there were no
dropouts from the study. This was probably due to several
reasons such as that the initial detoxification took place in
an inpatient basis assuring a high compliance with all
treatment interventions, that the study population was
highly motivated to withdraw from alcohol, and finally
that our sample consisted of individuals with relatively
high initial withdrawal symptoms who are usually
excluded from most studies of outpatient populations.
Impulsive and compulsive behaviours play a crucial
role in alcohol abuse, craving and relapse [39-41]; there-
fore, medications with anticraving properties have been
used for prevention of relapse. Several studies have
demonstrated topiramate’s efficacy in the management of
impulsive, aggressive and self-harmful behaviour [42],
gambling [43], eating disorders [44], as well as an adjunct
to SSRIs in obsessive-compulsive disorder [45]. Thus,
moderation of impulsivity with consequent minimization
of craving might be responsible for the lower rates of
relapse in the topiramate augmentation group. Moreover,
research has consistently documented a strong associa-
tion between anxiety and/or symptoms of depression and
alcohol abuse; these symptoms usually subside following
a few weeks of abstinence [46]. However, mild anxiety
[11] and minor symptoms of depression may persist for
several months and various medicines - including tiaga-
bine [47], mirtazapine and venlafaxine [12] - have been
used adjunctively to standard alcohol detoxification treat-
ment in order to increase patient compliance and
improve treatment outcome [10,48]. Through such a col-
lateral beneficial action, topiramate could lead to the
more favourable outcome observed in the augmentation
group of the present study.
The main limitations of the present study are: a) the
relatively small sample size, which reduces the statistical
significance of our findings, b) the study did not follow a
double-blind placebo control design; such a design was
not feasible due to the ethical restrictions of our institu-
tion, c) assessment of alcohol use during the follow-up
period was mostly based on self-reports and periodically
cross-checked with an informant and g-GT measurements,
and d) a longer follow-up period would provide important
information on the long term efficacy of topiramate in a
community setting. Despite the above limitations, our
results corroborate previous reports that show the poten-
tial usefulness of topiramate in the treatment of alcohol
dependence even when administered at low doses.
Conclusions
In conclusion, low-dose topiramate when used as an
adjunct to psychotherapy is well tolerated and effective
in reducing alcohol craving, as well as symptoms of
depression and anxiety, present during the early phase
of alcohol withdrawal. Furthermore, topiramate consid-
erably helps to abstain from drinking during the first
16-week post-detoxification period, a period which is
critical for relapse. Thus, topiramate could be an alter-
native option beyond the already approved agents for
the treatment of alcohol dependence.
Abbreviations
GABA: γ-Aminobutyric acid; DSM-IV-TR: Diagnostic and Statistical Manual of
Mental Disorders, Fourth Edition, Text Revision; SCAN: Schedules for Clinical
Assessment in Neuropsychiatry; γ-GT: γ-glutamyl transpeptidase; CIDI:
Composite International Diagnostic Interview; HDRS: Hamilton Depression
Rating Scale; HARS: Hamilton Anxiety Rating Scale; OCDS: Obsessive
Compulsive Drinking Scale; GAS: Global Assessment Scale; CIWA-Ar: Clinical
Institute Withdrawal Assessment for Alcohol, revised; SAFTEE: Systematic
Assessment for Treatment Emergent Events; RMANOVA: repeated measures
Table 3 Reported adverse effects by study group
Topiramate group
N = 30 (%)
Control group
N = 55 (%)
Dizziness 6 (20.0) 4 (7.2)
Somnolence 7 (23.3) 3 (5.4)*
Nervousness 2 (6.6) 7 (12.7)
Numbness/Paresthesias 3 (10.0) 3 (5.4)
Psychomotor slowness 4 (13.3) 2 (3.6)
Nausea 5 (16.6) 2 (3.6)
* Fisher’s Exact Test (2-tailed sig.), p < 0.05.
Paparrigopoulos et al.BMC Psychiatry 2011, 11:41
http://www.biomedcentral.com/1471-244X/11/41
Page 5 of 7



![Báo cáo seminar chuyên ngành Công nghệ hóa học và thực phẩm [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250711/hienkelvinzoi@gmail.com/135x160/47051752458701.jpg)






















