
Fish & Shellfish Immunology (2008) 25,1e18
available at www.sciencedirect.com
journal homepage: www.elsevier.com/locate/fsi
REVIEW
What happens to the DNA vaccine in fish?
A review of current knowledge
*
Tom Christian Tonheim , Jarl Bøgwald, Roy Ambli Dalmo **
Department of Marine Biotechnology, The Norwegian College of Fishery Science,
University of Tromsø, N-9037 Tromsø, Norway
Received 12 November 2007; revised 11 March 2008; accepted 12 March 2008
Available online 19 March 2008
KEYWORDS Abstract The primary function of DNA vaccines, a bacterial plasmid DNA containing a con-
DNA vaccine; struct for a given protective antigen, is to establish specific and long-lasting protective immu-
Fish; nity against diseases where conventional vaccines fail to induce protection. It is acknowledged
Tissue distribution; that less effort has been made to study the fate, in terms of cellular uptake, persistence and
degradation, of DNA vaccines after in vivo administration. However, during the last year some
Plasmid DNA receptors
papers have given new insights into the fate of DNA vaccines in fish. By comparing the newly
acquired information in fish with similar knowledge from studies in mammals, similarities with
regard to transport, blood clearance, cellular uptake and degradation of DNA vaccines have
been found. But the amount of DNA vaccine redistributed from the administration site after
intramuscular administration seems to differ between fish and mammals. This review presents
up-to-date and in-depth knowledge concerning the fate of DNA vaccines with emphasis on tis-
sue distribution, cellular uptake and uptake mechanism(s) before finally describing the intra-
cellular hurdles that DNA vaccines need to overcome in order to produce their gene product.
ª 2008 Elsevier Ltd. All rights reserved.
DNA vaccines capable of being replicated autonomously in prokaryotes.
Plasmid DNA used in gene delivery studies normally
contains promoter- and enhancer sequences, the gene of
A plasmid DNA (pDNA) is often used as a vehicle for gene interest, a poly-adenylation sequence, transcriptional
delivery to mammals and fish. The pDNA is a circular termination sequence, antibiotic resistance gene and origin
molecule consisting of double-stranded deoxyribonucleic of replication. To express the gene of interest, pDNA is
acid (DNA, not different from chromosomal DNA), usually transcribed and mRNA is translated to protein by the cell’s
own apparatus. This opens up the applicability of pDNA in
two important areas: gene therapy and DNA vaccination.
* Corresponding author. Tel.: þ47 77 6460 22; fax: þ4777 64 6020. The definition of gene therapy and DNA vaccines is not
** Corresponding author. Tel.: þ47 776444 82; fax: þ47 77 64 6020.consistent in the literature. Often, gene therapy also
E-mail addresses:tom.tonheim@nfh.uit.no(T.C. Tonheim),roy. encompasses the use of DNA vaccines. The Norwegian
dalmo@nfh.uit.no(R.A. Dalmo).
1050-4648/$ - see front matter ª 2008 Elsevier Ltd. All rights reserved.
doi:10.1016/j.fsi.2008.03.007

2 T.C. Tonheim et al.
Biotechnology Advisory Board ( www.bion.no ) defines gene lymphocytes [29]. In fish, the different subsets of T cells
therapy in animals as the intentional transfer of genetic are not well characterised, but CD8 [23,30e32] and CD4
material to somatic cells for purposes other than in uenc- [33e35] homologues are reported. Genes of MHC class IIb
ing the immune system [1]. Gene therapy is often aimed [36e38] and MHC class I gene [39] are found, and genes
to achieve a long-lasting physiologically matched expres- involved in MHC class I [40] and class II [41] loading path-
sion of the gene, without activating the immune system. ways are described in fish. Only three classes of immuno-
In contrast, DNA vaccination is defined as the intentional globulins; IgM [42e46] , IgD [47e54] and IgZ/IgT [55,56]
transfer of genetic material to somatic cells for the are reported in fish, but there are no indications that this
purposes of in uencing the immune system [1]. For DNA repertoire of immunoglobulins are functionally retarded
vaccination, a short-term expression is sufficient for evok- in any waydbesides the absence of, e.g., IgG, IgA and IgE
ing an immune response. in fish.
Immune responses are harder to investigate in fish
Working mechanism of DNA vaccines compared to mammals, due to lack of tools (e.g. cell
lines, gene sequences, markers and antibodies). However,
The immune response after DNA vaccination is primarily a recent publication shows that both innate and adaptive
launched by antigen presenting cells (APC), e.g. dendritic cell-mediated immune responses involving natural killer
cells (DC) [2]. Professional APC such as macrophages and DC (NK) cells and cytotoxic T lymphocytes (CTLs), respec-
have been shown to contain pDNA after i.m. administration tively, are triggered after a viral hemorrhagic septicaemia
[3,4] . Following on, the APC at the site of administration virus (VHSV) infection [9]. In rainbow trout, up-regulation
may prime immune cells such as nai ¨ve T lymphocytes fol- of MHC class II expression is found at the site of pDNA ad-
lowing antigen presentation [5]. APC containing cytosolic ministration [57], and expression of these genes is indica-
encoding pDNA may transcribe and translate the transgene tive of the recruitment of activated immunocompetent
and thereby produce immunogenic proteins, mimicking an cells (e.g. B cells and macrophages) [58]. The CpG sites
infection of an intracellular (cytosolic) pathogen and allow are regions of DNA where a cytosine nucleotide occurs
presentation of the foreign antigen peptide by major histo- next to a guanine nucleotide and is linked by a phospho-
compatibility complex (MHC) class I on the surface of the diester bond. These CpG sequences (CpG motifs) are nor-
APC. Antigen presenting cells can also take up soluble anti- mally highly methylated in mammals, but bacterial and
gens released from another transgene-producing cell (e.g. viral DNA possess a lower methylation frequency. As
a myocyte), process it and present the peptide on MHC class such, CpG sequences are often unmethylated in a pDNA
II molecules at the cell surface. The T-cell receptor (TCR) due to its bacterial origin. The vertebrate immune system
may recognise these peptides presented on the MHC class recognises unmethylated CpG-motifs as ‘‘foreign’’ and
I and MHC class II, which stimulate a CD 8 T-cell (cytotoxic ‘‘danger’’ signal. Cells and animals treated with synthetic
þ
CpG oligodeoxynucleotides (ODN) and pDNA may produce
T cell) and a CD4 T-cell (helper T cell) response, respec-
þ
cytokines that may induce macrophage activation, B-cell
tively. In addition, APC can also take up antigen-laden
proliferation and immunoglobulin secretion [59,60] . The
apoptotic bodies of transfected myocytes (or other cells)
and present the relevant peptides on MHCs ( Fig. 1 ). CpG DNA can also directly activate monocytes, macro-
Under certain circumstances, exogenously derived phages and dendritic cells to secrete Th1-like cytokines
peptides may be presented on both MHC I and II. This [60e62] to induce a cellular immune response, which is
cross-priming stems from the observation that professional the desired response for combating intracellular patho-
APC could present antigen or peptides from the classical gens. Leukocyte activation by CpG DNA is not mediated
exogenous pathway via the MHC class I pathway of antigen through a cell surface receptor but depends on uptake
presentation [6]. In contrast to the TCR restricted recogni- of the DNA [59] into lysosomal compartments harbouring
tion of MHC class I/II, the B lymphocyte, the precursor of specific receptors, the so-called Toll-like receptor 9
the antibody secreting cells, may directly recognise native (TLR9), for recognition [63,64] . TLR9 is expressed in den-
antigen through their B-cell receptor [5,6] . dritic cells [63], macrophages [63], B cells [65] and liver
One of the unique features of DNA vaccines is their endothelial cells [66]. TLRs function as innate pattern rec-
ability to stimulate both cellular (including cytotoxicity) ognition receptors (PRRs) and induce certain intracellular
and humoral immune responses [2,7e9] (Fig. 1 ). The cellu- signalling cascades that result in activation and transcrip-
lar immune response is represented, for simplicity, by the tion of pro-in ammatory genes [67e69] . A ligand for TLR9
activated Th1 cells that may secrete pro-in ammatory is CpG-containing pDNA and CpG ODNs. The CpG induced
cytokines, and CD8 T cells that may kill cells presenting immune responses seem to occur without apparent ad-
þ
the transgene. The adaptive humoral immune response is, verse effects [70]. Thus, it may be beneficial for a plasmid
however, represented by activation of B lymphocytes and vector to also include CpG motifs to increase and direct
antibody production. the immune response. Not all CpG-motifs possess the
Several findings suggest that the fish immune system same immune stimulatory activities, and the effects
resembles the mammalian immune system; homologies are depend on the ODN sequence, length, concentration and
found between mammalian and fish MHC class I (reviewed the recipient species [71]. As TLR9 has been proposed to
bind to CpG ODN, there are indications that also CpG-
in [10]), TCR[11e22] and the TCR co-receptor CD8 [23] sug-
containing pDNA bind to TLR9 [72,73] . However, it is not
gesting similarities in the antigen presentation process. The
clear whether the immunostimulatory CpG sequences in
lymphocytes detected in fish are equivalent to the mamma-
lian T and B cells [24e28] . However, the teleost (bony fish) the backbone of pDNA behave similarly to that predicted
B cells possess phagocytic activity, unlike the mammalian B from studies of synthetic CpG ODN [74].
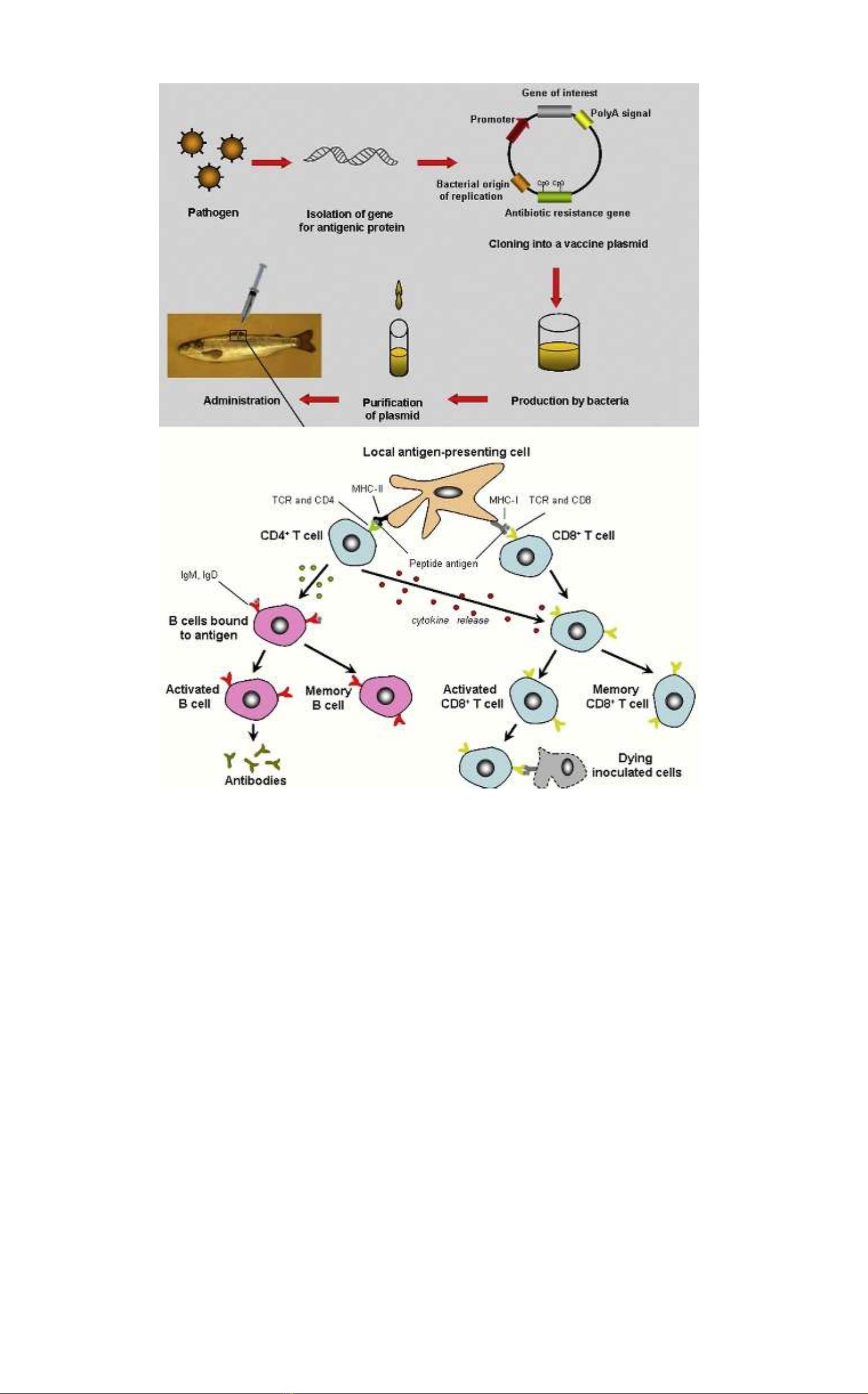
DNA vaccine in fish 3
Figure 1 A general and simplified overview of the adaptive immune response. DNA vaccines that have been taken up by, e.g.,
muscle cells and processed may be completely degraded or may be expressed by the muscle cells. The transfected cells produce
and release the plasmid encoding antigens that will be subjected to uptake in APC (antigen presenting cells). APC present
peptides,
being the breakdown products of the antigens, on MHC I and II to CD8and CD4cells following uptake of endogenously and exog-
þ þ
enously derived antigens, respectively. Activation of nai¨ve CD4cells will initiate cellular and humoral adaptive responses such as
þ
activation of B cells to secrete antibodies specific to this antigen, whereas binding of the T-cell receptor of CD8cells to MHC I
þ
presented peptides, with aid of cytokines produced by Th1 cells, will facilitate a cytotoxic T-cell response eliminating, e.g.,
virus-infected cells producing and expressing the virus antigen. One feature of the adaptive immune response is the generation
of memory cells that need short time to be activated during a later infection with the same pathogen. CD4, cluster of
differentiation
4 co-receptor; CD8, cluster of differentiation 8 co-receptor; MHC-I, major histocompatibility complex class I protein; MHC-II,
major
histocompatibility complex class II protein; IgM, immunoglobulin M; IgD, immunoglobulin D; TCR, T-cell receptor; Th1, T
helper 1.
Adapted from Online publication in Veterinary Sciences Tomorrow (www.vetscite.org/publish/articles/000020/index.html).
The TLR9 gene is found in fish[75,76]and is expressed in immune responses similar to that of mammals, such as
several epithelial and lymphoid tissues[77], but it remains activation of macrophages[78e81], proliferation of leuko-
to be elucidated whether pDNA binds to fish TLR9. cytes [79,82,83]and stimulation of cytokine expression
However, CpG ODN administered to fish, exert various[80,84,85].

4 T.C. Tonheim et al.
Advantages of DNA vaccines being tested clinically, rarely integrate [95,110,111] and
have a low probability of creating a tumorigenic event
[112]. However, attempts to enhance vaccine efficacy by
A huge advantage of DNA vaccination is that pDNA, like live
modifying the vector, co-administration of vaccine with
or attenuated viruses, effectively induces both humoral and
agents that increase cellular uptake or altering the site or
cell-mediated immune responses [86]. Immune responses to
method of delivery, may increase the potential for integra-
antigen present in soluble form, such as for recombinant
tion [105,113] . Homologous recombination could occur
protein, generally induce mainly antibody responses. In ad-
when the foreign DNA possesses a sequence that is very sim-
dition, DNA vaccines overcome the safety concerns com-
ilar to a sequence of the genomic DNA and the two se-
pared to live attenuated vaccines where reversion to
quences ‘‘cross over’’, although random insertion is
virulent forms may occur. Another benefit compared to con-
a more frequent event [114]. It should be emphasised that
ventional vaccination strategies is that pDNA possesses in-
such downside effects have never been found in fish. Envi-
trinsic immunostimulatory capacity, due to the presence
ronmental release of pDNA may occur by several ways,
of CpG motifs [87]. Furthermore, it is possible to construct
e.g. leakage of DNA vaccine from the administration site af-
a vector encoding several antigens to be given in a single ad-
ter vaccination (mainly in fish); by consumption of pDNA res-
ministration, and thus create a vaccine against multiple dis-
idues in the meat of DNA vaccinated animals; and by spills or
eases. From a practical point of view, the DNA vaccine is
waste of DNA vaccine from the production process. After
relatively inexpensive and easy to produce, and can be man-
vaccination pDNA may find its way to the intestine [115]
ufactured using identical production processes. DNA vac-
where pDNA may be taken up by intestinal bacteria or se-
cines are very stable as dried powder or in solution, unlike
creted by the faeces. Antibiotic resistance genes may then
conventional vaccines that often need to be stored under
be spread to various bacterial populations in the intestine,
proper conditions (e.g. cold environments) [58,88,89] .
soil or water. Several other advantages and disadvantages
DNA vaccines administered without any additional adju-
are listed in the review by Lorenzen and LaPatra [116].
vants create much less direct tissue damage or/and in am-
matory responses compared to conventional oil-adjuvanted DNA vaccine trials in fish
vaccines [90e94] , and with no systemic toxicity [95]. Per-
haps the most important use of DNA vaccine technology is
DNA vaccinations against a wide range of pathogens have
the possibility to create vaccines for targeted diseases
been investigated in various fish species ( Table 1 ). Consid-
where traditional vaccines have scored sub-optimally.
erable efforts have been made to develop conventional
Limitation and downside effects of DNA vaccines vaccines or subunit vaccines for many of these fish patho-
gens with limited success [117e119] . The IHNV and VHSV
Eukaryotic transcription and production of plasmid DNA are known to cause high mortalities of cultured salmonid
encoding a non-glycosylated protein is highly feasible, but species such as Pacific salmon (Oncorhynchus sp.), Atlantic
not the transcription and production of carbohydrates and salmon and rainbow trout in North America and Europe.
highly glycosylated proteins. This suggests that DNA vacci- Therefore, much effort has been concentrated around de-
nation cannot be a substitute for the more traditional veloping DNA vaccines against these viral diseases. Several
polysaccharide containing vaccines in evoking immune re- parameters have been investigated to optimise the efficacy
sponses against microbes that have an outer membrane of the DNA vaccines against IHNV and VHSV, such as dose
made of, e.g., lipopolysaccharides [89]. Following on, the and fish size [120e123] and optimal administration route
pDNA contains CpG moieties that may act as an adjuvant [124e126] . In short, i.m. administration seems to be the
[59,96,97] , andthere is a possibility that the combined adju- optimal route of administration for these DNA vaccines ( Ta-
vant activity with the presence of DNA may induce antibody ble 1 ) and it appears beneficial to vaccinate the fish when
responses to the DNA itself, but usually it does not [98e100] . they are small, since larger fish require a higher dose of
Such a process may be detrimental to the host, as exempli- vaccine [122,127]. Early protection involves short-lived,
fied by occurrence of glomerulonephritis in mice [101]. non-specific, anti-viral defence mechanisms, like induction
Whether there exists an antibody response to DNA in fish is of interferons and Mx proteins [126,128e130] , that may be
not known. An additional safety concern associated with important in protection against heterologous viruses (cross-
the use of DNA vaccines after i.m. administration is that my- protection) [129,131] However, the specific protection may
ocytes, taking up the pDNA and expressing the encoded an- last up to 2 years after i.m. administration [92].
tigen, may become targets for antigen-specific CTLs [102]. The glycoprotein (G-protein) of both IHNV and VHSV
This would result in the development of myositis. [132,133] is known as a strong inducer of neutralising and
Tolerance seems to be a problem in mainly neonatal protective antibodies. Intramuscular administration of
individuals, where an immature immune system could pDNA encoding the VHSV G-protein [134,135] or the IHNV
recognise the DNA vaccine encoded protein as a self protein G-protein [92,136] gives high levels of protection in rainbow
[103e105] . However, during neonatal DNA vaccination, tol- trout. However, other rhabdoviral proteins give limited or
erance is the exception rather than the rule [106]. The sus- no protection [134e138] . In addition, i.m. administered
ceptibility to tolerance induction was shown to wane within pDNA encoding IHNV G-protein also protects Atlantic
1 week after birth in mice and to increase with increasing salmon against IHNV infection [139]. Finally, in 2005 a po-
pDNA dose [107]. Although likely not to occur, integration tential prophylactic strategy against IHNV infection was in-
of pDNA into the chromosomal DNA could lead to mutations, troduced: The IHNV DNA vaccine (Apex-IHN ) developed by
genomic instability and abnormalities [105,108,109] . Avail- Aqua Health Ltd (an affiliate of Novartis), was cleared for
able evidence indicates that genetic vaccines, currently marketing by the Canadian Food Inspection Agency.

DNA vaccine in fish 5
Table 1 An overview of studies performed with DNA vaccines encoding viral or bacterial antigens in fish
Pathogen Pathogen gene in vaccine Host Administration Protection References
a b
routec
AHNV VHSV-G Turbot i.m. Yes [131]
Capsid protein (AHNV) Turbot i.m. No [131]
Capsid protein (AHNV) Turbot i.m. No [140]
CCV 7 genes (CCV) Channel catfish i.m. Yes [141]
HIRRV HIRRV-G Japanese ounder i.m. Yes [142]
IHNV IHNV-G Rainbow trout i.m. Yes [92,122,124,128e130,136,137]
IHNV-G Rainbow trout Gene gun Yes [124]
IHNV-G Rainbow trout i.p. Partial [124]
IHNV-G Rainbow trout Immersion No [124]
IHNV-G Rainbow trout Scarification No [124]
IHNV-G Rainbow trout i.b. No [124]
IHNV-G Atlantic salmon i.m. Yes [139]
IHNV-G Chinook salmon i.m. Yes [143]
IHNV-G Sockeye salmon i.m. Yes [143]
IHNV-G2stop Rainbow trout i.m. No [144]
IHNV-N, P, M, or NV Rainbow trout i.m. No [137]
SVCV-G Rainbow trout i.m. Yes [130]
SHRV-G Rainbow trout i.m. Yes [130]
IPNV ORF of Segment A (IPNV) Atlantic salmon i.m. Yes [145]
ISAV Hemagglutinin-esterase (ISAV) Atlantic salmon i.m. Yes [146]
Nucleoprotein (ISAV) Atlantic salmon i.m. No [146]
LCDV Capsid protein (LCDV) Japanese ounder i.m. Yes [147]
RSIV Capsid protein (RSIV) Red seabream i.m. Yes [148]
SVCV SVCV-G Common carp i.m. Yes [149]
VHSV VHSV-G Rainbow trout i.m. Yes [121,126,127,129,135,138]
VHSV-G Rainbow trout i.p. Partial [126]
Nucleocapsid protein (VHSV) Rainbow trout i.m. Yes [135]
Nucleocapsid protein (VHSV) Rainbow trout i.m. No [138]
Aeromonas Omp38 gene, Omp48 gene Spotted sand bass i.m. Partial [150]
veronii
Mycobacterium Ag58A gene Hybrid striped bass i.m. Yes [151,152]
marinum Ag58A gene Hybrid striped bass i.p. Partial [151]
Piscirickettsia Full expression library Coho salmon i.m. Partial [153]
salmonis
Renibacterium Aeromonas salmonicida DNA Rainbow trout i.m. No [154]
salmoninarum p57 Rainbow trout i.m. Yes [154]
Vibrio OMP38 gene Asian seabass i.m. Yes [155]
anguillarum
Atlantic salmon (Salmo salar), Asian seabass (Lates calcarifer), channel catfish (Ictalurus punctatus), chinook salmon (Oncorhynchus
tshawytscha), coho salmon (O. kisutch), common carp (Cyprinus carpio), hybrid striped bass (Morone saxatilis M. chrysops), Japanese
ounder (Paralichthys olivaceus), rainbow trout (Oncorhynchus mykiss), red seabream (Pagrus major), sockeye salmon (Oncorhynchus
nerka), spotted sand bass (Paralabrax maculatofasciatus), turbot (Scophthalmus maximus).
aPathogens: AHNV, Atlantic halibut nodavirus; CCV, channel catfish virus; HIRRV, hirame rhabdovirus; IHNV, infectious haematopoietic
necrosis virus; IPNV, infectious pancreatic necrosis virus; ISAV, infectious salmon anemia virus; LCDV, lymphocystis disease virus; RSIV,
red seabream iridovirus; SVCV, spring viraemia of carp virus; VHSV, viral haemorrhagic septicaemia virus.
bPathogen genes: G, glycoprotein; e.g. SHRV-G, snakehead rhabdovirus glycoprotein.
c Administration routes: i.m., intramuscular; i.p., intraperitoneal; i.b., intrabuccal; scarification, scarification of the skin.
Distribution of plasmid DNA pDNA by endonucleases at the administration site, and
(iv) distribution of pDNA by blood, cells and lymph to
various tissues.
Mammals Bureau and co-workers have suggested that as soon as
pDNA is administered, it is proportionally partitioned
When pDNA (DNA vaccine) is i.m. administered to mam- between at least two compartments. While a major part
mals, several events other than the intended immune of the pDNA is cleared and degraded, a very small amount
response are initiated: (i) uptake of pDNA by cells at the of the administered pDNA is located in another compart-
administration site, (ii) pDNA remaining extracellularly ment where it is protected from endogenous nucleases
located at the administration site, (iii) degradation of




![Tổng hợp 5-[Substituted]-1, 3, 4-thiadiazol-2-amines: Phân tích đặc tính quang phổ và đánh giá tương tác DNA](https://cdn.tailieu.vn/images/document/thumbnail/2020/20200525/tocectocec/135x160/1001590394742.jpg)




![Hướng dẫn giải chi tiết bài tập phân li, phân li độc lập: Tài liệu [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251204/lethu2868@gmail.com/135x160/84711764814448.jpg)



![Bài tập Đa dạng thế giới sống [kèm đáp án/ hướng dẫn giải]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251123/thaohoang9203@gmail.com/135x160/5861763951302.jpg)










