
An experimental study of oil recovery from sewage sludge
by low-temperature pyrolysis in a fluidised-bed
q
Lilly Shen, Dong-Ke Zhang*
School of Chemical Engineering, Curtin University of Technology, GPO Box U1987, Perth, WA 6845, Australia
Accepted 9 September 2002; available online 22 October 2002
Abstract
Pyrolysis of activated sewage sludge was investigated under inert conditions in a fluidised-bed to study the effects of temperature and gas
residence time on the product distribution and composition with an aim to maximise the oil yield. The temperature was varied from 300 to
600 8C and the gas residence time from 1.5 to 3.5 s. Three groups of products were produced, namely, a non-condensable gas (NCG) phase, a
solid phase (char) and a liquid phase (oil). A maximum of 30% oil yield (wt% daf of sludge fed) was achieved at a pyrolysis temperature of
525 8C and a gas residence time of 1.5 s. Higher temperatures and longer gas residence times favoured the formation of NCG, suggesting that
secondary cracking reactions had occurred. The oil obtained was analysed using GC–MS and H NMR to determine the oil’s composition and
structure, a unit structure of the oil was proposed which consisted of aromatic rings connected by hydrocarbons with –OH functional groups
attached.
q2002 Elsevier Science Ltd. All rights reserved.
Keywords: Fluidised-bed reactor; Sewage sludge devolatilisation; Oil structure
1. Introduction
Sewage sludge like most organic wastes is abundant in
volatile matter and thus there exists an opportunity to
convert this stored energy into a usable energy source such
as for heating or perhaps as a liquid fuel substitute and the
production of speciality chemicals [1]. Presently, most of
the sewage sludge waste is disposed off in landfills, ocean or
via incineration [2,3]. The disposal of dewatered sewage
sludge in sanitary landfills is not very satisfactory because
of the inherent chemical energy lost and associated health
problems [4]. Ocean dumping disturbs, at least locally, the
ecology of the biosphere and should be avoided [5].
Incineration provides a large volume reduction of sewage
sludge and results in improved thermal efficiency. However,
the scrubbing costs of the product gases for air pollution
control are usually very high [3].
In the past decade, pyrolysis of sewage sludge in an
oxygen-free atmosphere at relatively low-temperatures (ca.
500 8C) has generated significant interest [6]. Particular
interest is shown in this process as a high recovery of liquid
oil is achieved, lower emissions of NO
x
and SO
x
also lower
operating costs when compared to incineration [7]. Tests
have shown that the oil obtained from sewage sludge
pyrolysis can be used directly in diesel fuelled engines and
is comparable to low-grade petroleum distillates from
commercial refineries [8].
Many researchers have investigated the effects of
operating parameters such as temperature and residence
time on product distribution [9,10]. However, many have
focused on fixed beds [11] and rotary reactors [9]. Very few
have performed low-temperature pyrolysis of sewage
sludge in fluidised-bed reactors [12]. In the few studies
that have used fluidised-bed reactors, the oil was lumped
together without further classification of their composition
or structure [13,14]. In this study a fluidised-bed reactor was
used to determine the oil recovery and product distribution
of sewage sludge under moderate temperatures (300–
600 8C) and varying gas residence time (1.5 –3.5 s). A
GC–MS was used to investigate the molecular distribution
and structure of the oil and H NMR analysis was used to
detect the type of functional groups in the oil and verify the
GC–MS results. H NMR analysis has been previously used
to determine tar composition from coal pyrolysis and has
shown to be useful in identifying the type of H-bonds in coal
derived oils [15].
0016-2361/03/$ - see front matter q2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 0 1 6 - 2 3 6 1 ( 0 2 ) 0 0 2 9 4 - 6
Fuel 82 (2003) 465–472
www.fuelfirst.com
q
Published first on the web via Fuelfirst.com_http://www.fuelfirst.com
*Corresponding author. Tel.: þ61-8-9266-7581; fax: þ61-8-9266-3554.
E-mail address: dkzhang@che.curtin.edu.au (D.K. Zhang).
2. Experimental
2.1. Sample preparation
An activated sewage sludge from Western Australia was
used for this study to observe the effects of pyrolysis
temperature and gas residence time on product distribution
and their structures. Table 1 shows the analyses of the sewage
sludge sample. The dewatered sludge was crushed and sieved
to a particle size of 212– 355 mm and dried at 1058C for 24 h.
The dry sample was then kept in an airtight container to
prevent re-absorption of moisture before experimentation.
2.2. Experimental apparatus
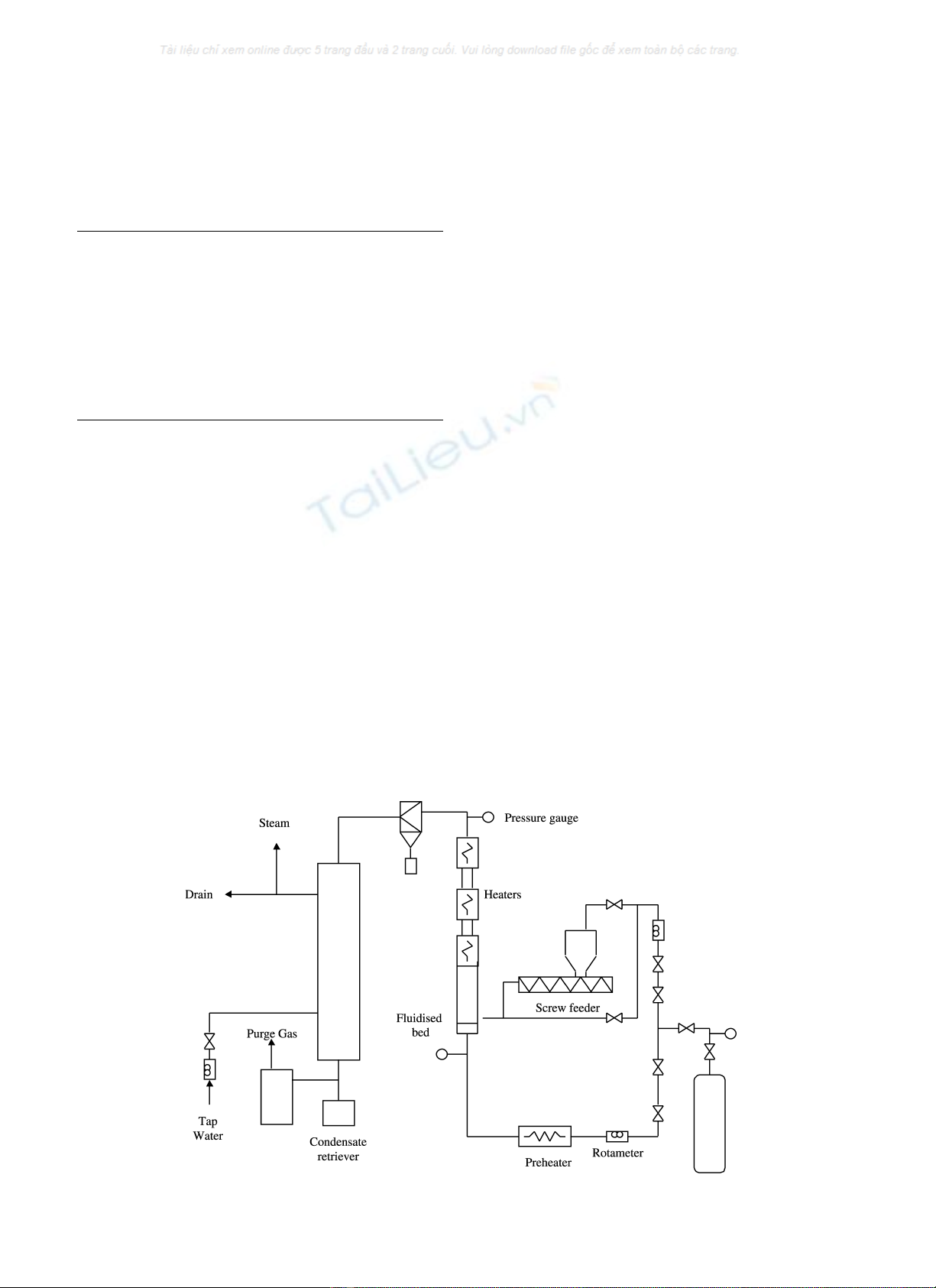
Fig. 1 shows the schematic of the apparatus in which
pyrolysis of the sewage sludge is continuously performed in
a fluidised bed with an internal diameter of 50 mm. The
sludge is fed from the sludge hopper by a screw feeder,
40 cm above the distribution plate where nitrogen is used as
the fluidising gas. Nitrogen is also fed through the hopper
and the nozzle of the screw feeder to assist the flow of the
solid. The product gas leaves from the top of the reactor and
through a cyclone the solid residual (char) is separated and
collected in a char container situated below the cyclone. The
product gas is then condensed in a shell-and-tube stainless
steel condenser to ambient temperature using tap water as
the cooling medium. The condensed oil product is collected
in a container below the condenser and the non-condensable
gases (NCG) are sampled through a gas trap.
PLC controllers using a visual basic interface through a
PC, controls the process. The computer stores all the data
from sensors, such as, thermocouples and pressure trans-
mitters. The heating is provided by electric means as the
usage of NCG to provide the process heat is not feasible on
this scale, however, is an alternative for larger scale plants.
A pre-heater providing 230 W is used to heat the fluidising
gas to a pre-set temperature between 300 and 600 8C. The
pre-heater is supplied by a 15 A-power source with ceramic
beads packed into the heater for better heat transfer. Three
other heaters, each providing 120 W with 10 A are used in
the reactor to compensate the heat loss through the reactor
wall.
2.3. Experimental procedure
During an experimental run, the sludge hopper was filled
with sewage sludge and the fluidised bed was loaded with
quartz sand in the size fraction of 400– 555 mm (Umf:
Table 1
Proximate and ultimate analyses of the activated Western Australian
sewage sludge
Proximate analysis (wt%)
Moisture (as fed) 3
Ash (as fed) 22.6
Volatile matter (daf) 61.3
Fixed carbon (daf) 16.1
Ultimate analysis (wt% daf)
C 41.8
H 5.4
N 4.3
S 1.14
O, Cl (by diff.) 47.36
Fig. 1. A schematic diagram of the fluidised-bed pyrolysis reactor apparatus.
L. Shen, D.-K. Zhang / Fuel 82 (2003) 465–472466
42 mm s
21
at 400 8C). Nitrogen, flowing at five times the
minimum fluidising velocity was turned on to purge
the apparatus from gaseous residues and oxygen. Mains
tap water was turned (at 10 l min
21
) on to provide the
cooling medium for the condenser. The operating par-
ameters, such as temperature (300 –600 8C), feed rate of
sludge (3.3–4 g min
21
) and fluidising velocity were set by
the operator using the PC and the signals were then sent to
the controllers. After the system reached steady state the
screw conveyor (runs by a variable speed motor rotating at
50–120 rpm) is automatically switched on and the sludge is
uniformly fed to the reactor. The process was terminated by
stopping the feed and by controlled cooling of the apparatus.
Material balances were taken of sludge consumed and all
products collected. It was found that the material balances
are generally within 92 –95%.
Oil samples were retrieved from the apparatus with
acetone. The cyclone, condenser and other equipment where
oil may have deposited were washed with acetone as soon as
the experiment was finished to recover the maximum
amount of volatile released. The oil is analysed by a GC–
MS operating at 300 8C for 40 min so that sufficient
separation of the liquid could be achieved. The GC–MS
was able to determine the compounds and their molecular
structure in the oil ranging from C
4
–C
40
. The GC– MS has
an extensive library and the chromatograms of the
compounds in the oil were compared with the compounds
in the library. The library then generates all the information
available of the compounds, including their structures and
molecular weights. H NMR analysis was also performed on
the oil samples to determine the type of functional groups in
the oil. Gas samples were taken in 30 min intervals with
2–3 samples taken during a run from the gas-trap and
analysed using a GC. Gas yields were calculated from the
gas analysis and the total gas flow through the reactor at the
time of sampling. Averages of the samples were taken and
are reported in this paper. The operating conditions are also
summarised in Table 2.
3. Results and discussion
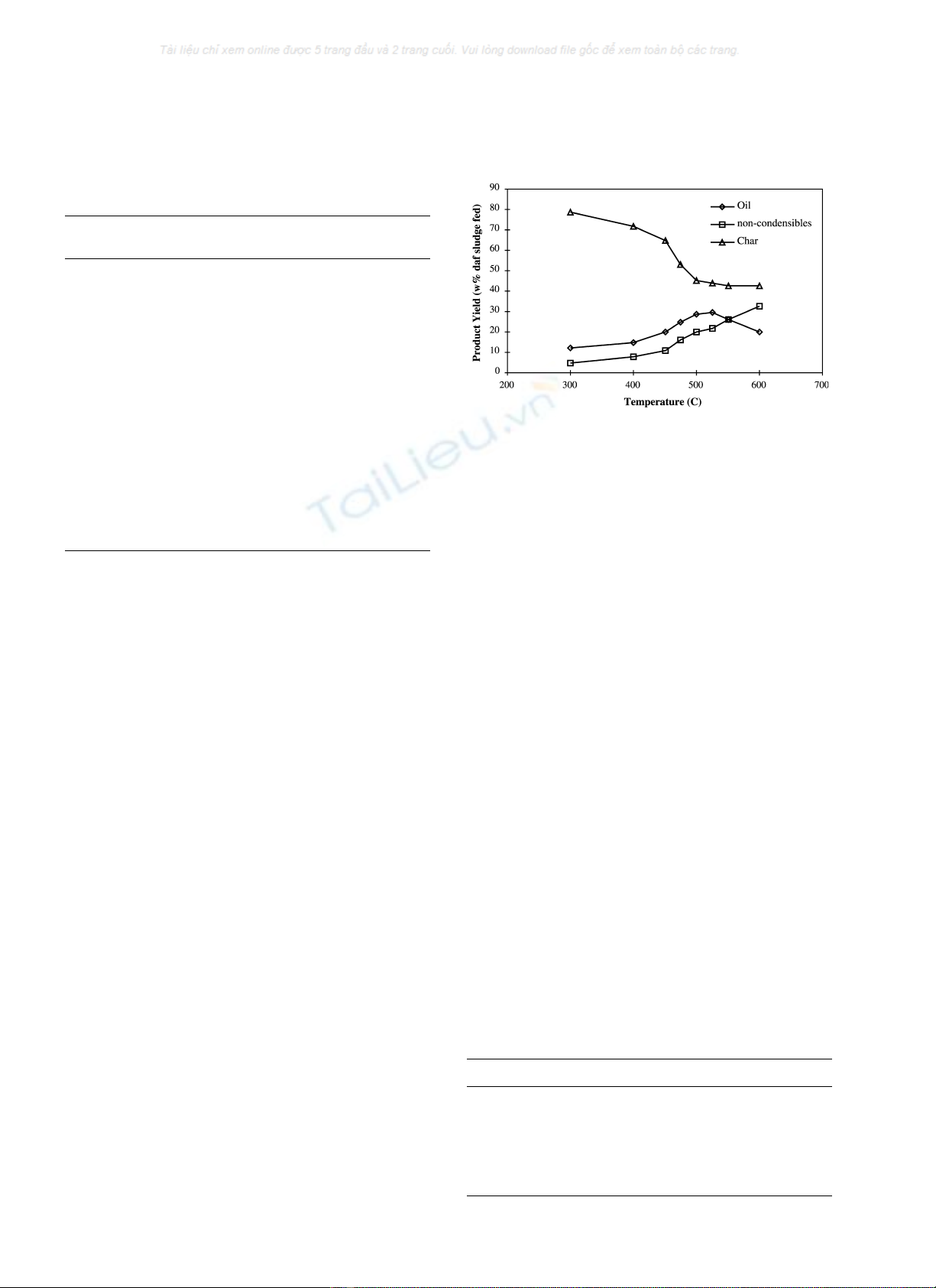
Fig. 2 shows the effect of pyrolysis temperature on oil,
char and NCG yields with a constant gas residence time of
1.5 s. The maximum oil yield obtained was 30% daf of the
feed at 525 8C. In order to understand this trend, organic
bonds that might have been broken at different temperatures
during the pyrolysis were obtained from the literature [16,
17] and summarised in Table 3. From Table 3 it can be seen
that at the temperature range of 500–575 8C carboxylic,
phenolic, ether oxygen and cellulosic bonds are broken [16,
17]. The temperature range, however, for maximum oil
yield is on the upper end of the temperature band for
decomposition of carboxylic and phenolic groups. Above
600 8C most of the carboxylic and phenolic bonds have been
broken and this is when the oil yield is observed to drop.
Suggesting that carboxylic and phenolic breakage from
Table 2
Summary of operating conditions for the experiments conducted
Test no. Pyrolysis temperature
(8C)
Test duration
(min)
Gas residence time
(s)
1 302 88 1.5
2 401 91 1.5
3 451 90 1.5
4 476 86 1.5
5 502 89 1.5
6 526 93 1.5
7 552 87 1.5
8 601 89 1.5
9 524 92 2.0
10 527 86 2.5
11 525 87 2.75
12 523 93 3.0
13 522 94 3.5
14 301 87 2.0
15 298 90 2.5
16 302 93 2.75
17 301 96 3.0
18 297 89 3.5
Fig. 2. Effect of bed temperature on product yields of oil, NCG and char for
sewage sludge expressed in percentage weight daf basis of sludge fed with a
constant gas residence time of 1.5 s.
Table 3
Temperature ranges for different groups of compounds to decompose
Groups of compounds decomposed Temperature range (8C)
Moisture [16] up to 150
Carboxylic [16] 150–600
Phenolic [16] 300–600
Ether oxygen [16] up to 600
Cellulosic [17] up to 650
Oxygen containing compounds [16] 150– 900
L. Shen, D.-K. Zhang / Fuel 82 (2003) 465–472 467
the feed generates the oil. Table 3 was obtained for brown
coal, however, this information is not yet available for
sludge. Thus this information can only be indicative of the
possible bonds that might also be present in the sludge [18,
19]. The char yield, on the other hand, decreased steadily
with increasing temperature, while the NCG yield increased
continuously.
The oil yields increase with increasing temperature
initially as sludge is subjected to more energy, stronger
bonds break and thus an increase in larger compounds are
observed. The decrease in oil yields above 525 8Cis
believed to be a result of secondary decomposition reactions
which break the oil into lighter, gaseous hydrocarbons. As a
consequence the NCG yields also increase. Char yields are
expected to decline as more volatiles are released. These
results are similar to the literature observations made in both
coal decomposition and wood decomposition [13,20– 22].
By convention used for coal oils [23], heavy oils are
defined as species with molecular weight greater than
150. In determining the quality of the oil produced, the
selectivity of the light oils is of great importance. In
most cases, light hydrocarbons are preferred as they can
be easily used as fuels. It was found that the species with
MW less than 150 in the oil consisted of compounds
with carbon numbers less than 9 and of aromatic nature.
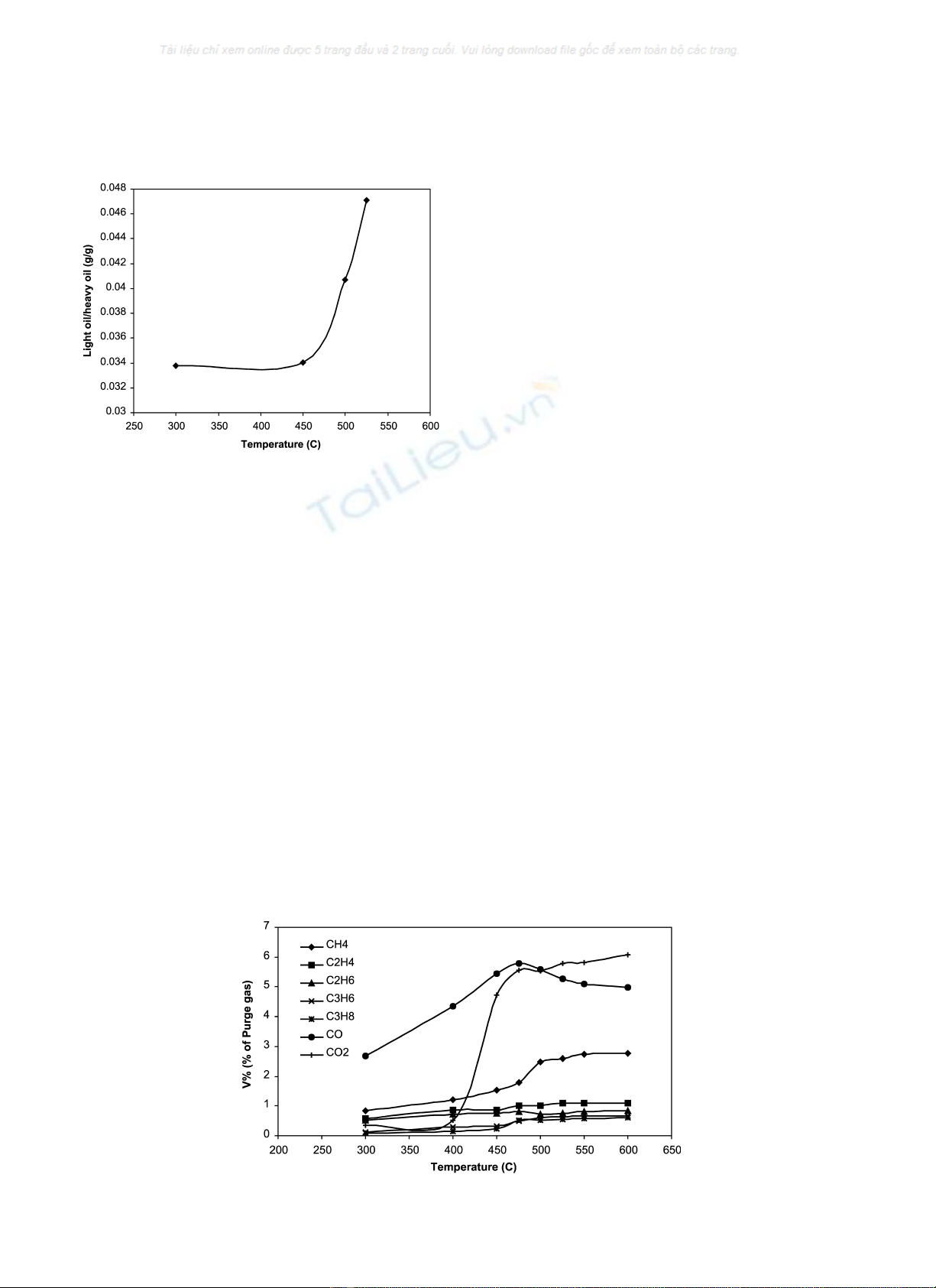
Fig. 3 shows the selectivity of light oil to heavy oil for
different temperatures. The selectivity of light oils
increase significantly after 450 8C and stays relatively
constant before 450 8C. Therefore, it suggests that the
rate of secondary cracking reactions is only significant
after 450 8C at short residence times.
Fig. 4 shows the effect of pyrolysis temperature on
different NCG expressed as volume percent of purge gas,
gas detected after the condenser in Fig. 1. The gases
detected by the GC included CH
4
,C
2
H
4
,C
2
H
6
,C
3
H
6
,C
3
H
8
,
CO and CO
2
. Nitrous oxide (N
2
O) was also detected,
however, the maximum volume percent is 131 ppm at
600 8C and thus the trend cannot be represented clearly on
Fig. 4. The yields of all the gases increased steadily with
temperature with the exception of CO, a behaviour that is
typical of the pyrolysis of peat, lignite as well as cellulosic
biomass [23–26]. It can be observed that methane has the
highest yield, while propylene and propane the lowest
among all hydrocarbons. Carbon dioxide has the highest
yield of 6.3% of the non-hydrocarbon gases at a 600 8C.
Horne and William [24] has found similar concentrations of
CO and CO
2
under similar operating conditions, where CO
and CO
2
were found to be 6.8 and 6.7%, respectively, for a
biomass. Presently, there is no available data in the literature
for nitrous oxide yields for comparison. However, it has
been found that N
2
O is generally formed in reducing
atmospheres and low temperatures in fluidised bed reactors
[27]. This coincides with the operating conditions of this
study.
Fig. 3. The selectivity of light oils to heavy oils obtained from sewage
sludge pyrolysis at different temperatures with a gas residence time of 1.5 s.
Fig. 4. Effect of bed temperature on NCG yields for sewage sludge expressed in percentage volume of purge gas with a residence time of 1.5 s.
L. Shen, D.-K. Zhang / Fuel 82 (2003) 465–472468
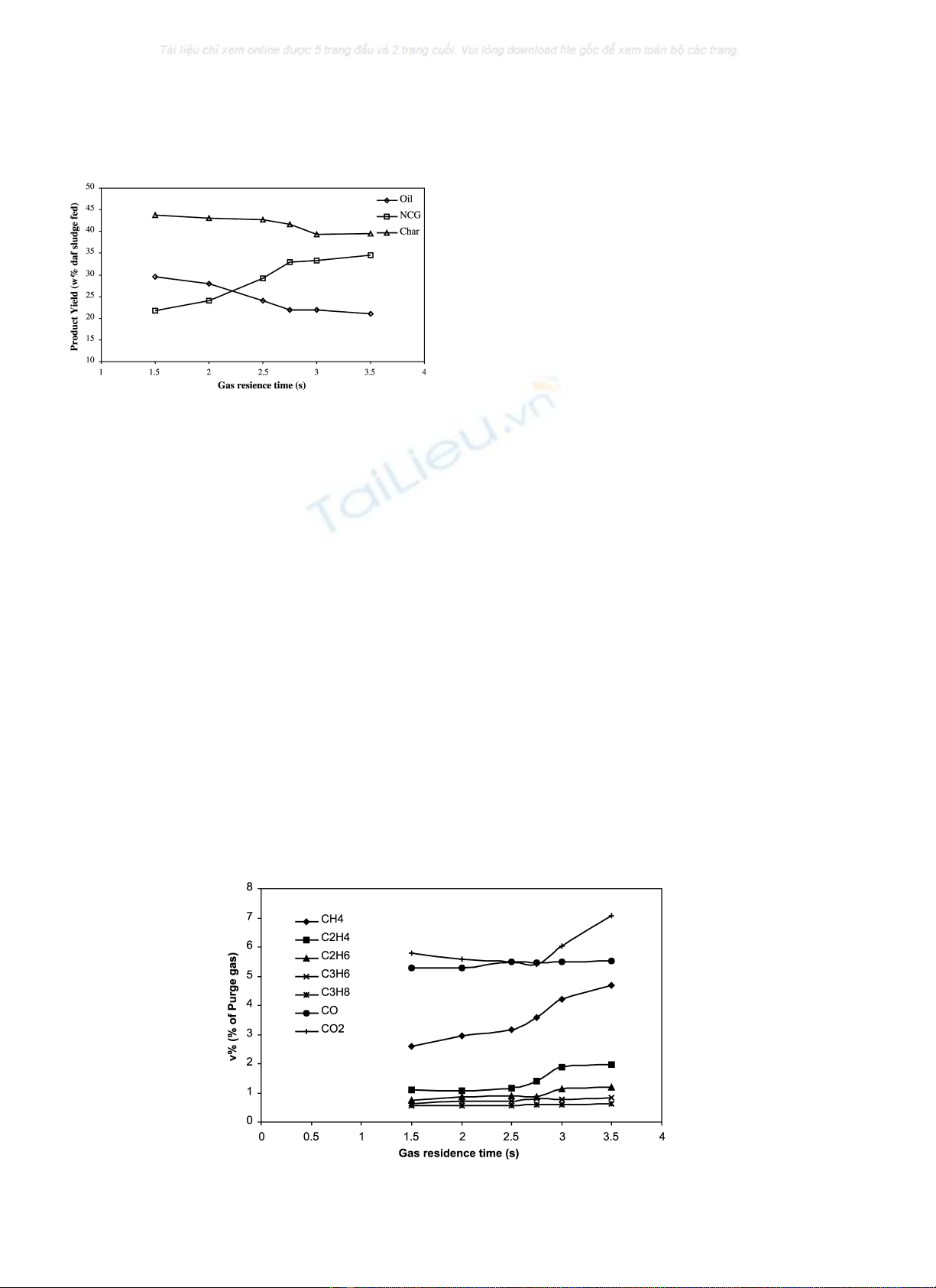
The effect of gas residence time was also studied and
the trends of product yields are shown in Fig. 5 for a
constant pyrolysis temperature of 525 8C. Oil and char
yields are both at maximum at the shortest residence
time of 1.5 s. This result is in agreement with other work
carried out with a variety of biomass and coal feeds [28,
29]. The decrease of char yield is accompanied by
evolution of more volatile matter from the sewage
sludge. Oil yields decrease from a maximum of 30 to
21% (daf of feed), which can be explained by
the occurrence of secondary cracking reactions, breaking
up larger species in the volatiles evolved. Henceforth the
yields of NCG increase.
The NCG represents all the gases identified in the gas
trap. The effect of gas residence time on C
1
–C
3
hydro-
carbons, CO and CO
2
are presented in Fig. 6. The maximum
yield of N
2
O is found to be 92 ppm at 3.5 s. This trend is not
presented in Fig. 6 as the values are too small in scale.
All the gas species increase with increasing gas residence
time and the rate of increase is similar for all gases. Methane
again has the highest conversion while propane has the
lowest out of the hydrocarbons and CO
2
again is the
dominant gas. CO
2
had a maximum yield of 7.2%. After
2.75 s the conversions of the hydrocarbons level off. This
may be associated with secondary decomposition in the
light hydrocarbons itself, where propane is broken down to
smaller species. This phenomenon has been observed in
previous studies where C
3
hydrocarbons start to decrease
with increasing pyrolysis temperature and residence time, as
seen in both coal [26] and biomass [21] pyrolysis.
When the yields of all the products are calculated, 92–
95% daf basis of the feed mass can be accounted for. The
discrepancy is a result of many factors and one of the major
reasons is that a portion of solid char is lost along with the
NCG due to the inability of the cyclone. Also at the
operating temperatures used in the experiment, light
hydrocarbons in the oil would be expected to evaporate as
soon as it was formed. When compared to previous studies
[24], the total products accounted for compared favourably.
The molecular distributions of the species in oil obtained
at varying temperature and gas residence time are shown in
Fig. 7. In this figure, peaks are seen at a molecular weight of
360. When the gas residence time increases, the curve shifts
towards the left, suggesting that smaller species are formed
when the time increases, apparently a result of secondary
reactions occurring.
Table 4 shows all the species that had been identified,
by the GC– MS, in the oil at 525 8C. Not all of the
compounds were identified in all of the experiments
conducted. For example, at 300 8C the heavier compounds
(MW .700) were not detected as shown in Fig. 7. These
identified compounds were made up of aromatic rings
linked by long straight hydrocarbon chains. Table 5 shows
Fig. 6. Effect of gas residence time on NCG yields for sewage sludge expressed in percentage volume of purge gas at a constant pyrolysis temperature of
525 8C.
Fig. 5. Effect of gas residence time on product yields of oil, NCG and char
for sewage sludge expressed in percentage weight daf basis of sludge fed at
a constant pyrolysis temperature of 525 8C.
L. Shen, D.-K. Zhang / Fuel 82 (2003) 465–472 469



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





